| |
|
|
ROLE
OF STATINS IN PCOS
AUTHOR:
Pankaj D. DESAI MD (O&G)
Chief of Unit in Obgyn (VRS)
Medical College and S.S. G. Hospital Baroda
Co-Author
Dr. Munjal Pandya
Assistant Professor
AMC MET Medical College
Sheth L. G. Hospital
Ahmedabad
INTRODUCTION:
Polycystic ovarian syndrome (PCOS) is a combination of
various symptoms due to an imbalance in hormonal
homeostasis. Main features seen in a patient with PCOS are
the result of high androgen levels, along with disturbances
in lipoprotein equilibrium. Previously known as the
Polycystic Ovarian Disease (PCOD), has various pathological
changes, thus replacing the word ‘Disease’ with ‘Syndrome’.
Also known as “Stein Leventhal Syndrome”, it has various
defining criteria according to various societies. According
to the National Institute of Health Criteria,
hyperandrogenism and oligo/amenorrhoea are required to stamp
a case as of PCOS.1 Rotterdam criteria consists
of fulfilling of any two of three criteria (hyperandrogenism,
oligo/amenorrhoea, polycystic ovaries).2 Androgen
Access Society (2006) recommended the presence of clinical
and/or biochemical hyperandrogenism and either oligo/anovulation
or polycystic ovarian morphology.3 Hormonal
profile of PCOS patients has derangements like high
androgens, relatively increased estrogens, reduced Sex
Hormone Binding Globulin (SHBG), and high insulin levels.
Co-morbidities of PCOS
High
circulating insulin levels make subjects with PCOS more
prone to development of gestational as well as type II
diabetes. Patients with PCOS are more prone to develop an
atherosclerotic disease, as compared to the common
population. Low-density lipoproteins (LDL), triglycerides,
and Very Low-Density Lipoproteins (VLDL) are higher while
High-Density Lipoproteins (HDL) levels are lower in patients
with PCOS.4 The risk of myocardial infarction is
more in these patients owing to increased size and stiffness
of the left ventricle along with increased homocysteine
levels, increased androgen levels and increased chances of
calcification of coronary arteries.3, 5 These
patients are more prone to develop metabolic syndrome, along
with obesity and propensity to develop diabetes.6
Chronic
anovulation in PCOS patients makes them susceptible to
endometrial hyperplasia, which may advance to endometrial
adenocarcinoma. PCOS patients have hyperplasia of
theca-interstitial cells, caused by increased insulin as
well as oxidative stress, leading to hyperandrogenism.7
It is believed that this increased insulin level is
responsible for hyperandrogenism, by increased production
from theca-interstitial cells, as well as reduced apoptosis
of the same.8, 9 Increased insulin levels also
inhibit SHBG, thus increasing unbound free androgen levels.10
Oxidative
stress has been proved to be instrumental in deranging
homeostasis in subjects with PCOS, by increased generation
of Reactive Oxygen Species (ROS), which leads to more of
systemic inflammation, even in lean patients.11
Insulin and systemic inflammatory cells are proved to
increase oxidative damage independently, inducing theca cell
proliferation.12, 13, 14 Oxidative stress also
causes disturbances in insulin signalling, thus stimulating
more insulin secretion, making it a vicious cycle.
Pathophysiology involved in PCOS has been explained as an
algorithm in Fig. 1
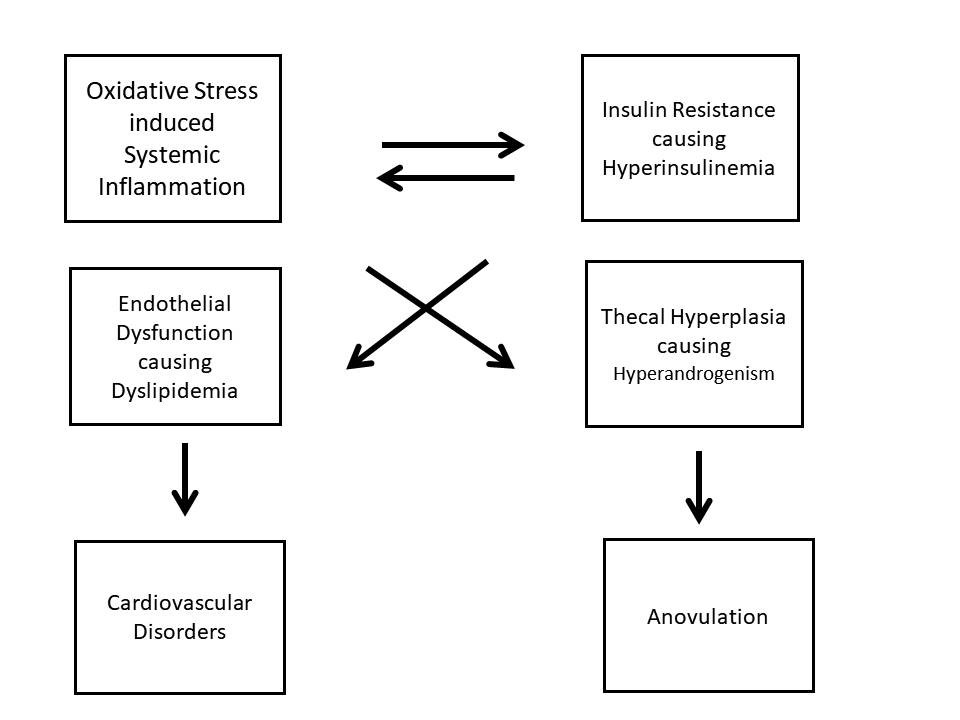
Fig. 1
Pathophysiology of PCOS
What are Statins?
Any of group of drugs which act to
reduce levels of cholesterol in the blood are called statins.
They are also known as HMG-CoA reductase inhibitors, are a
class of lipid-lowering medications. Also known as
hypolipidemic agents, or antihyperlipidemic agents, they are
a diverse group of pharmaceuticals that are used in the
treatment of high levels of fats (lipids), such as
cholesterol, in the blood (hyperlipidemia). They are called
lipid-lowering drugs. z
Statins have variety of effects,
beneficial for PCOS patients, which include endothelial
function improvement, increased nitric oxide, anti-oxidant
effect, reduction in inflammatory markers and
immunomodulation. Statins are used for improving lipid
profile (reducing LDL), thus will be helpful in PCOS
patients. High testosterone levels also decline with their
usage, an added advantage offered.15, 16, 17, 18
However; studies have shown little improvement with
menstrual irregularity and with hirsutism with statin
monotherapy.
Mechanism of action of statins
The Mevalonate pathway is the all critical pathway in the
action of statins. Mevalonate pathway causes the conversion
of acetyl-CoA into isopentenyl pyrophosphate, the essential
building block of all isoprenoids. It is also known as the
isoprenoid pathway or HMG-CoA reductase pathway and is an
essential metabolic pathway present in eukaryotes, archaea,
and some bacteria. The pathway produces two five-carbon
building blocks called isopentenyl pyrophosphate (IPP) and
dimethylallyl pyrophosphate (DMAPP), which are used to make
isoprenoids, a diverse class of over 30,000 biomolecules
such as cholesterol, heme, vitamin K, coenzyme Q10, and all
steroid hormones.
It produces mevalonate from
Hydroxymethylglutaryl-CoA (HMG-CoA), the former being an
essential product for cholesterol synthesis. (Fig. 2) The
rate-limiting enzyme for this pathway is HMG-CoA Reductase,
which is reversibly inhibited by statins, thus improving the
lipid profile in PCOS patients.15 Inhibition of this enzyme
leads to reduced levels of dolichol, geranyl-geranyl
pyrophosphate (GGPP) and farnesyl pyrophosphate (FPP).
Dolichol is essential for maturation of insulin and
insulin-like growth factor-1 (IGF-1) receptors, so its
reduction is helpful for PCOS patients.19 GGPP
and FPP have an important role in post-transitional
modification of GTPase proteins, which have an essential
role in cellular mechanics.20 Reduced levels of
these proteins, thus, inactivates signal transduction of
mitotic activity, decreasing tissue growth, along with a
reduction in oxidative stress. The anti-oxidant effect seems
to be due to NADPH oxidase activity inhibition as well as
inhibition of oxidized LDL production and anti-free radicle
action.21, 22
Statins are proved to have lowered LDL
cholesterol by reducing its synthesis as well as by its
clearance, along with improving HDL and triglyceride levels.
Inflammatory markers are seen to be reduced as well.16, 17
Statins have been effective in activating AMP-activated
protein kinase (AMPK), which is important for cellular
metabolic and energy homeostasis.
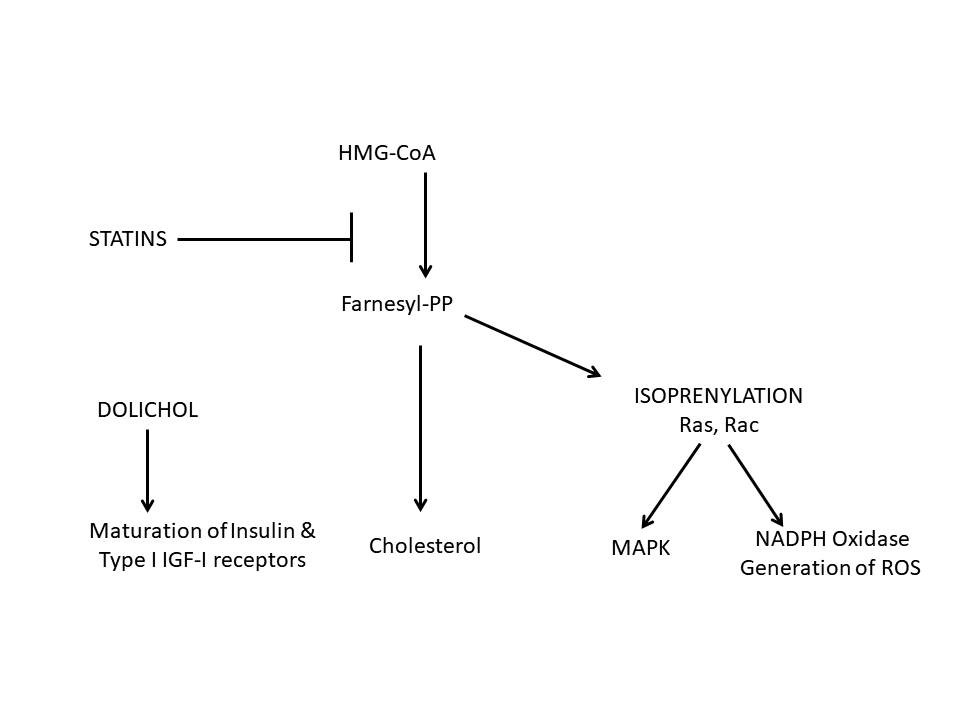
Fig 2: Mechanism of action
of statins
Hyperandrogenism
Hirsutism is one of the most
distressful features, contributing to psychological turmoil
of hyperandrogenism. Statins have been promising agents in
various studies with a reduction in testosterone level,
improvement in LH: FSH ratio, reduction in ovarian size.23
Cholesterol levels are reduced with statins, which again is
a component for androgen production, thus correcting
hyperandrogenism.
Polycystic ovaries
Statins have been effective in reducing
the ovarian size, as well as in improving ovarian cycle.
Improvement in LH: FSH ratio, along with a reduction in LH
level contributes to unifollicular maturation, thus
regularizing menstrual cycle. Insulin and IGF-1 actions on
the ovary are also limited.
Obesity and Insulin resistance
PCOS patients usually have impaired insulin
sensitivity and statins in a majority of the studies showed
a reduction in insulin resistance. Reduced triglyceride
leads to more usage of glucose, improving insulin
homeostasis. Rosuvastatin showed worsening of insulin
sensitivity. 24
Pre-treatment with atorvastatin,
followed by metformin usage, has proved to be of a
synergistic effect with improvement in metabolic parameters
and inflammatory markers. The study showed that 3 months
treatment with atorvastatin followed by 3 months treatment
of metformin leads to accumulative 33% reduction in insulin
and 35% reduction in HOMA-IR.
Cardiovascular risk
Improvement in the lipid profile,
inflammatory markers with statin usage reduce chances of
atherosclerotic risk in PCOS patients.
Clinical Studies
Various studies have been done with
statin alone, as well as with oral contraceptive pills (OC
Pills), with metformin.
Simvastatin
Randomised controlled trials performed
with one group on simvastatin with OC Pills and the other
group on OC Pills alone, showed improvement in lipid
profile, with reduction in Luteinizing Hormone (LH) level,
testosterone level, inflammatory markers and hirsutism in
the former group.25, 26 A trial using metformin,
simvastatin, and combination showed the results as:
reduction in cholesterol was more in patients who received
simvastatin (alone and in combination groups), reduction in
testosterone was better in simvastatin alone group,
improvement in menstrual irregularity was more with
simvastatin group. 23 One more study divided
patients into two groups, one receiving simvastatin and
metformin combination and the other one receiving metformin
with placebo. Reduction in testosterone, LDL, total
cholesterol, LH, hirsutism was noted in former group.27
Atorvastatin
Atorvastatin was found to have reduced
inflammatory marker high sensitivity C-reactive protein (hs-CRP),
which is a predictor of cardiovascular events.28
hsCRP reduction also reduces insulin resistance in
pre-diabetics. A trial comparing simvastatin with
atorvastatin showed a reduction in testosterone,
homocysteine, fasting insulin, LDL and LH levels in both
groups. Reduction in homocysteine levels was much greater in
atorvastatin group as compared to simvastatin group.29
Mevastatin
Mevastatin inhibits theca-interstitial
proliferation and androgenesis.30 Mevastatin has
been found to be having an inhibitory effect on mesenchymal
cells, including smooth muscle, myocytes, mesangial cells.31,
32, 33, 34, 35 Mevastatin and simvastatin have been
found to inhibit NADPH oxidase subunits effectively, thus
decreasing steroidogenesis.36 OC Pills have been
very promising in improving SHBG when given for at least 3
months, much more effective than statins alone.
Adverse effects of statins:
Headache, sleep disturbances,
drowsiness, nausea, vomiting, abdominal pain, bloating,
constipation, skin rash are the known side effects of
statins. Infrequent complications like liver toxicity may
occur in patients with acute liver disease. Risk of diabetes
may increase and few studies also reported reversible
dementia with its usage.37
Teratogenesis of statins: Statins have
been rated as High-risk Category agents, thus making it
compulsory for patients to use contraceptive methods along
with their use. Though, statin-induced teratogenicity risk
is small.
Conclusion
As PCOS is being diagnosed rampantly in
today’s era, along with lifestyle and dietary modifications,
Statins as potentially promising agents need to be given
attention for their inclusion in routine usage guidelines,
so as to benefit patients with more fruitful outcomes, to
their maximum satisfaction.
References
1. ACOG Committee on Practice
Bulletins--Gynecology. ACOG Committee on Practice Bulletin
No. 108: Polycystic ovary syndrome. Obstet Gynecol.
2009;114:936–49
2. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop
Group. Revised 2003 consensus on diagnostic criteria and
long-term health risks related to polycystic ovary syndrome.
Fertil Steril. 2004;81:19–25
3. Azziz R, Carmina E, Dawailly D, et al. Position
statement: Criteria for defining polycystic ovary syndrome
as a predominantly hyperandrogenic syndrome: an Androgen
Excess Society guideline. J Clin Endocrin Metab.
2006;91(11):4237–45
4. Rizzo M, Berneis K. Who needs to care about small, dense
low-density lipoproteins? Int J Clin Pract. 2007;61:1949–56
5. Sirmans SM, Weidman-Evans E, Everton V, Thompson D.
Polycystic ovary syndrome and chronic inflammation: pharmaco
therapeutic implications. Ann Pharmacother. 2012;46:403–18
6. Apridonidze T, Essah PA, Iuorno MJ, Nestler JE.
Prevalence and characteristics of the metabolic syndrome in
women with polycystic ovary syndrome. J Clin Endocrinol
Metab. 2005;90:1929–35
7. Nelson VL, Legro RS, Strauss JF et al. Augmented androgen
production is a stable steroidogenic phenotype of propagated
theca cells from polycystic ovaries. Molecular
Endocrinology. 1999;13(6):946-957
8. Dunaif A, Green G, Futterweit W, Dobrjansky A.
Suppression of hyperandrogenism does not improve peripheral
or hepatic insulin resistance in the polycystic ovary
syndrome. J Clin Endocrinol Metab. 1990;70:699–704
9. Nestler JE, Barlascini CO, Matt DW, et al. Suppression of
serum insulin by diazoxide reduces serum testosterone levels
in obese women with polycystic ovary syndrome. J Clin
Endocrinol Metab. 1989;68:1027–32
10. Nestler JE, Powers LP, Matt DW, et al. A direct effect
of hyperinsulinemia on serum sex hormone-binding globulin
levels in obese women with the polycystic ovary syndrome. J
Clin Endocrinol Metab. 1991;72:83–9
11. Yilmaz M, Bukan N, Ayvaz G, et al. The effects of
rosiglitazone and metformin on oxidative stress and
homocysteine levels in lean patients with polycystic ovary
syndrome. Hum Reprod. 2005;20(12):3333–40
12. Adamson GM, Billings RE. Tumor necrosis factor induced
oxidative stress in isolated mouse hepatocytes. Arch Biochem
Biophys. 1992;294:223–9
13. Krieger-Brauer HI, Kather H. Human fat cells possess a
plasma membrane-bound H2O2 generating system that is
activated by insulin via a mechanism bypassing the receptor
kinase. J Clin Invest. 1992;89:1006–13
14. Rifici VA, Schneider SH, Khachadurian AK. Stimulation of
low-density lipoprotein oxidation by insulin and insulin
like growth factor I. Atherosclerosis. 1994;107:99–108
15. Kodaman PH, Duleba AJ. Statins: Do they have potential
in the treatment of polycystic ovary syndrome? Semin Reprod
Med. 2008 Jan; 26(1): 127–138
16. Sathyapalan T, Atkin SL. Evidence for statin therapy in
polycystic ovary syndrome. Ther Adv Endocrinol Metab.
2010;1:15–22
17. Ferri N, Corsini A. Clinical evidence of statin therapy
in non-dyslipidemic disorders. Pharmacol Res. 2014;88:20–30
18. Raval AD, Hunter T, Stuckey B, Hart RJ. Statins for
women with polycystic ovary syndrome not actively trying to
conceive. Cochrane Database Syst Rev. 2011;(10):CD008565
19. Carlberg M, Dricu A, Blegen H, et al. Mevalonic acid is
limiting for N-linked glycosylation and translocation of the
insulin-like growth factor-I receptor to the cell surface.
Evidence for a new link between 3-hydroxy-3-methylglutaryl
coenzyme A reductase and cell growth. J Biol Chem.
1996;271:17453–62
20. Zhang FL, Casey PJ. Protein prenylation: molecular
mechanism and functional consequences. Ann Rev Biochem.
1996;65:241–69
21. Wassmann S, Laufs U, Muller K, et al. Cellular
antioxidant effects of atorvastatin in vitro and in vivo.
Arterioscler Thromb Vasc Biol. 2002;22:300–5.
22. Franzoni F, Quinones-Galvan A, Regoli F, Ferrannini E,
Galetta F. A comparative study of the in vitro antioxidant
activity of statins. Int J Cardiol. 2003;90:317–21
23. Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ.
Comparison of simvastatin and metformin in treatment of
polycystic ovary syndrome: prospective randomized trial. J
Clin Endocrinol Metab. 2009;94:4938–45
24. Ghazeeri G, Abbas HA, Skaff B, Harajly S, Awwad J.
Inadequacy of initiating rosuvastatin then metformin on
biochemical profile of polycystic ovarian syndrome patients.
J Endocrinol Invest. 2015;38:643–51
25. Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L.
Simvastatin improves biochemical parameters in women with
polycystic ovary syndrome: results of a prospective,
randomized trial. Fertil Steril. 2006;85:996–1001
26. Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J,
Duleba AJ. Effects of simvastatin and oral contraceptive
agent on polycystic ovary syndrome: prospective randomized
cross-over trial. J Clin Endocrinol Metab. 2007;92:456–61
27. Kazerooni T, Shojaei-Baghini A, Dehbashi S, Asadi N,
Ghaffarpasand F, Kazerooni Y. Effects of metformin plus
simvastatin on polycystic ovary syndrome: a prospective,
randomized, double-blind, placebo-controlled study. Fertil
Steril. 2010;94:2208–13
28. Raja-Khan N, Kunselman AR, Hogeman CS, Stetter CM,
Demers LM, Legro RS. Effects of atorvastatin on vascular
function, inflammation, and androgens in women with
polycystic ovary syndrome: a double-blind, randomized,
placebo-controlled trial. Fertil Steril. 2011;95:1849–52
29. Kaya C, Cengiz SD, Berker B, Demirtaş S, Cesur M,
Erdoğan G. Comparative effects of atorvastatin and
simvastatin on the plasma total homocysteine levels in women
with polycystic ovary syndrome: a prospective randomized
study. Fertil Steril. 2009;92:635–42
30. Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ.
Mevastatin inhibits ovarian theca-interstitial cell
proliferation and steroidogenesis. Fertil Steril.
2004;82:1193–7
31. O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG
coenzyme A reductase inhibitor, improves endothelial
function within 1 month. Circulation. 1997;95:1126–31
32. Axel DI, Riessen R, Runge H, Viebahn R, Karsch KR.
Effects of cerivastatin on human arterial smooth muscle cell
proliferation and migration in transfilter cocultures. J
Cardiovasc Pharmacol. 2000;35:619–29
33. Buemi M, Allegra A, Senatore M, et al. Pro-apoptotic
effect of fluvastatin on human smooth muscle cells. Eur J
Pharmacol. 1999;370:201–3
34. El-Ani D, Zimlichman R. Simvastatin induces apoptosis of
cultured rat cardiomyocytes. J Basic Clinical Physiol
Pharmacol. 2001;12(4):325–38
35. Danesh FR, Sadeghi MM, Amro N, et al.
3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent
high glucose-induced proliferation of mesangial cells via
modulation of Rho GTPase/p21 signaling pathway: implications
for diabetic nephropathy. Proc Natl Acad Sci USA.
2002;99:8301–5
36. Piotrowski P, Kwintkiewicz J, Rzepczynska I, Duleba AJ.
Simvastatin and mevastatin inhibit expression of NADPH
oxidase subunits: p22phox and p47phox in rat
theca-interstitial cells; 52nd Annual Meeting of the Society
for Gynecologic Investigation; Los Angeles, CA. 2005.Mar
23-26
37. Thompson PD, Panza G, Zaleski A, Taylor B. Statin-associated
side effects. J Am Coll Cardiol. 2016;67:2395–410 |
|

