|
Congenital
Malformations of
Uterus and Reproduction
Pankaj
Desai, Sameer Dixit
INTRODUCTION
Developmental
abnormalities of the Müllerian system are one of the most
fascinating conditions in gynecology. A wide range of
abnormalities occur, ranging from mild to severe. Müllerian
abnormalities are associated with renal and axial skeletal
abnormalities and management also involves investigations
and treatment of these conditions.
The earliest reported case of Müllerian abnormality is in
16th century.1 The actual incidence of the Müllerian
abnormalities is unknown. The true incidence may never be
known as these are grossly under reported in childhood and
over reported in the population undergoing infertility
treatment. The incidence varies widely and depends on the
type of study. Most authors report an incidence varying from
0.1–3.5 percent. The most accepted incidence is 4.3 percent
for a general population and fertile women, 3.5 percent for
infertile women and 13 percent in women with recurrent
pregnancy loss.2 In a study of women undergoing Müllerian
assessment at the time of undergoing tubal ligation, an
incidence of 3.2 percent was identified.3
The incidence
also varies with the type of diagnostic test used. In a
study4 of women undergoing HSG for recurrent pregnancy loss,
the incidence was 8–10 percent. With emergence of more
sensitive diagnostic tests, standardized classification and
aggressive management, a more realistic incidence of the
abnormalities would emerge in future.
EMBRYOLOGY OF THE FEMALE GENITAL TRACT
The
reproductive organs of a woman consist of the external
genitalia, the Müllerian tubes and the gonads. All three
develop from different primordial origins and in close
association with the urinary system and the hind gut. In
women, the Müllerian system develops over the Wolffian
system. The cranial parts of the Wolffian system persist as
epoophoron and the caudal parts as the Gartner’s duct. The
Müllerian system persists and forms the adult fallopian
tubes, the uterine corpus, the cervix and part of the
vagina.
At 37 weeks
after fertilization, the Müllerian ducts appear on either
side of the Wolffian duct as invagination of the dorsal
celomic epithelium. The site of the origin remains patent as
the fimbrial end of the Fallopian tubes. The paired
Müllerian tubes grow caudally and medially until they meet
together in the midline and become fused together. They then
converge on the urogenital septum.
The solid
Müllerian tubes undergo simultaneous canalization to form
the tubular structures. The cranial parts which remain
patent and separate evolve into the Fallopian tubes. The
upper fused portions of the tubes form the uterus while the
lowermost fused portion forms the cervix. The vagina is
formed from both the lower end of the fused Müllerian ducts
and the urogenital sinus. The mesenchyme surrounding the
Müllerian ducts condenses to form the musculature of the
female genital tract.
Congenital
abnormalities of the female genital tract occur due to
errors in this developmental process.5 These include:
• Agenesis/Hypoplasia
• Abnormal fusion with urogenital sinus
• Abnormal lateral fusion of the paired Müllerian tubes
• Abnormal resorption of the septum of the fused Müllerian
tubes
Developing kidney and urinary system closely follows
Müllerian system. So, their abnormalities are closely
associated with abnormalities of Müllerian system.
Disruption of developing local mesoderm and somites accounts
for some axial skeletal abnormalities. Cardiac and auditory
defects are occasionally associated. Ovarian morphogenesis
is distinct from Müllerian organogenesis. Hence, in these
conditions, the ovarian function and hormonal milieu is
normal.
GENETICS OF
MÜLLERIAN
ABNORMALITIES
The abnormalities occur due to disruption of the
morphogenesis. Various factors6 have been implicated in
this. These include:
1. Abnormal intrauterine milieu
2. Teratogens like DES and Thalidomide
3. Genetic factors
Genetic
factors involved are complex. They commonly occur
sporadically. If familial incidence is detected then it is
usually multifactorial. Other modalities of inheritance like
autosomal dominant, autosomal recessive and X-linked
disorders also exist.6 Müllerian anomalies may also
represent a part of the multiple malformation syndromes.7
Associated
anomalies are usually seen in cases of Müllerian aplasia (AFS
class I). Associated urological anomalies range between
15–40% in these cases and skeletal anomalies (congenital
absence or fusion of vertebrae) occur in 12–50% of these
cases.8 An association between MRKH syndrome and
Klippel-Fiel syndrome is reported. This syndrome is
characterized by congenital fusion of the cervical
vertebrae, a short neck, low posterior hairline and limited
range of motion in cervical spine.9 The MURCS association (Müllerian
duct aplasia, Unilateral Renal aplasia, Cervicothoracic
Somite dysplasia) is another variant. Infrequently auditory
deficits and cardiac defects can be found. The karyotype of
the females having Müllerian aplasia is 46 XX.
Müllerian
agenesis has been associated with variants of
galactose-1-phosphate uridyltransferase (GALT) enzyme. This
finding suggests that increased exposure to galactose is
responsible for abnormal Müllerian development.10
Other theories about genetic disorders include abnormalities
in MIS (Müllerian Inhibitory Substance) gene, loss of
function mutation in WNT4 gene and abnormalities of
HOXA9-HOXA13 genes. However, none of these have been
consistently associated.
TECHNIQUES
TO ASSESS THE FEMALE GENITAL TRACT
History
In case of MRKH syndrome, the patient may present with
primary amenorrhea. In cases of a bicornuate uterus, patient
may complain of dysmenorrhea. In cases of a vertical vaginal
septum, the patient may come with the complaint that a
tampon is unable to stop menstrual flow. In case of an
obstructive vaginal septum, the patient may complain of
dyspareunia.
Clinical
Examination
Per speculum examination may reveal vaginal septum or
duplication of cervix. Bimanual examination may help in
identifying another horn.
Hysterosalpingography
This has been the classical tool for diagnosis of the
uterine anomalies (Figure 1). When the classic picture of
the uterus with two fallopian tubes is seen, Müllerian
abnormalities are almost ruled out.
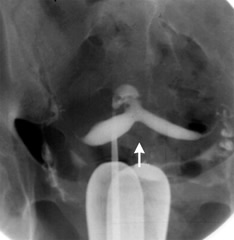
Figure 1 HSG
image of bicornuate uterus
Standard Sonography
In routine
sonography, the key structure to be scrutinized is the
endometrial cavity. In a normal uterus, the endometrial
cavity is single. In a bicornuate uterus, two cavities are
appreciated (Figure 2). It must be noted that it is
difficult to appreciate two cavities in a longitudinal scan.
However, the clinching image is the transverse plane. The
two uterine cavities in a cross section are easily
identified. A TV sonography gives better diagnosis than the
TA sonography. The advantages of sonography are that it is
easily available; it is widely acceptable to the patients
and is relatively cheap. However, the drawbacks are that the
intracavitary lesions are not easily identified, a septum is
not appreciated well and it does not depict the external
contour of the fundus.
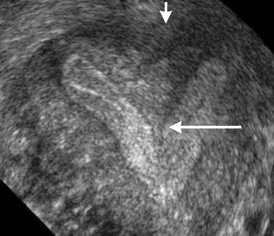
Figure 2 Bicornuate
uterus
Saline
Infusion Sonography (SIS)
SIS combines
the USG with HSG. A catheter is placed inside the
endometrial cavity and saline is instilled to achieve
separation of the walls of the endometrial cavity along with
continuous transvaginal ultrasound visualization. It is
usually performed within first 10 days of the cycle. It is
not performed after that period, firstly to avoid aborting
an early pregnancy and secondly, the thick secretary
endometrium may appear wrinkled to give false positive
impression of endometrial pathology.
A balloon
catheter like the pediatric Foley’s is inserted with tip
within the uterine cavity. The balloon is distended with 1ml
of water to prevent backflow of the saline. Slow
instillation of 3 to 10 ml of prewarmed saline is done.
Imaging with transvaginal sonography is done to visualize
separation of the uterine walls (Figure 3). Once adequate
distension is confirmed, a sagittal sweep from cornu to
cornu and an axial sweep from fundus to the cervix are done.
The balloon is then deflated and fluid allowed to flow out.
A second examination with empty uterus is performed. At the
end of the examination, free fluid in the pouch of Douglas
is looked for. Its presence denotes patency of at least one
fallopian tube.
Potential pitfalls include blood clots or mucous plugs
(created by shearing effect of the catheter) being mistaken
for intrauterine pathology or overdistension of the uterus
causing intrauterine pathology to be missed.
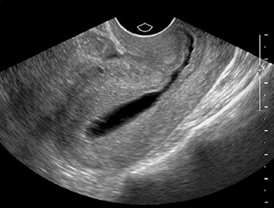
Figure 3 SIS
showing uterine cavity
3
Dimensional Sonography
Conventional 3D sonography or 3D sonography coupled with
saline infusion is better than 2D sonography (Figures 4 to
6). It involves rapid acquisition of data volumes which are
stored for subsequent analysis. Acquisition of the coronal
plane improves diagnosis in almost 30.8 percent of the
patients.11 This includes better evaluation of the external
uterine contour, detection of adhesions and identification
of intrauterine pathologies.
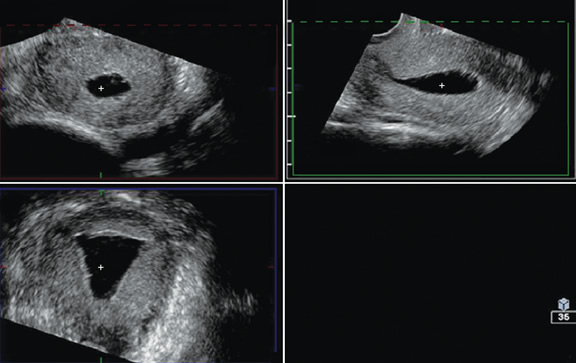
Figures 4A
to D Multiplanar rendering of 3D acquisition showing all
three planes
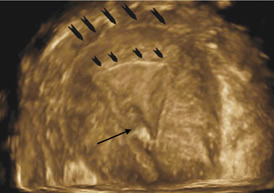
Figure 5 A
3D rendered image. Outer arrows point to the fundus, inner
arrows to endometrial cavity and single arrow to the
catheter tip
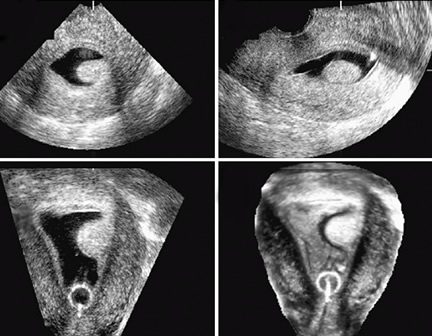
Figures 6A
to D 3D Sonography
Laparoscopy
Laparoscopy allows one to see the fundus of the uterus
from outside. The differentiation between the bicornuate
uterus and the septate uterus is made by the appearance of
the fundus. A septate uterus shows single convex fundus
while a bicornuate uterus shows two cornuae.
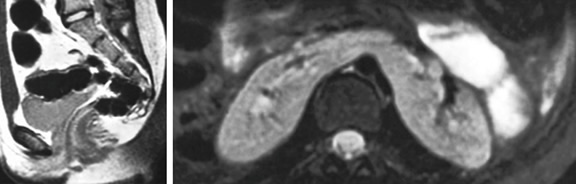
Figures 7 to 9
show Helical CT image of normal uterus, CT image of
bicornuate uterus with horse-shoe kidney, MRI image of
bicornuate uterus and hysteroscopic image of subseptate
uterus respectively.
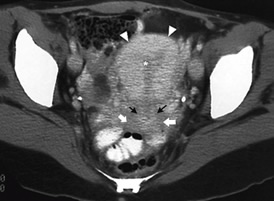
Figures
7 Helical CT image of normal uterus
Figures 8a
and B CT image of bicornuate uterus (A) with horse-shoe
kidney (B)
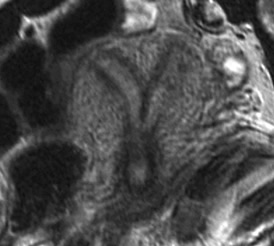
Figure 9 MRI
image of bicornuate uterus
ESHRE/ESGE
consensus on diagnosis of female genital anomalies (21)
Diagnostic Methods:
1. Clinical examination- An essential starting point and
essential part of the evaluation. It also offers unique
evaluation of vaginal and cervical abnormalities
2. HSG- It offers reliable information regarding uterine
anatomy in the absence of cervical obstruction. It can also
provide information regarding cervical can if it is patent.
It however, does not provide any information regarding
anatomy of vagina. It can not be used for diagnosing
obstructive abnormalities. Its efficacy is limited by false
positive and false negatives
3. 2D USG- It is a reliable, objective and measurable tool.
Its an essential part of the assessment. However, it is
dynamic, depends on experience of the clinician. It needs a
systematic approach.
4. Recommendations for proper use of 2D USG- The endometrial
line should always be visible for precise imaging of the
uterus. Serial sagittal and transverse scan should be taken
extending beyond the margins of the uterus.
5. Hysterosalpingo contrast sonography- Early follicular
phase is recommended to avoid pregnancy and artefacts due to
thick secretary endometrium.
6. 3D USG- It can provide highly reliable, objective and,
most importantly, measurable information for the anatomy of
the cervix, uterine cavity, uterine wall, external contour
of the uterus and for associated pelvic pathology; the
coronal plane of the uterus does provide a clear image of
the cavity and the external profile of the uterine fundus.
3D volumes give reliable and objective representation of the
examined organs more independently of the examiner
overcoming the limitations of obtaining coronal images with
2D sonography. It can provide, also, measurable information
even for obstructed parts of the female genital tract.
7. Recommendations for proper use of 3D USG- This method
should be started with a 2D evaluation of the uterus. Use in
mid cycle or luteal phase is encouraged as this demonstrates
the endometrial wall and the outline of the cavity at its
best. Contrast medium could be used for the evaluation of
the cavity and the tubes; in these cases, the examination
has to be performed in the early follicular phase. Save a 3D
volume for off-line analysis. The reconstructed coronal
plane of the uterus might show the cavity and the external
uterine profile as well as the tubal angle and the
junctional zone, if possible along all the endometrium and
cavity. Acquisition of an isolated cervical volume, without
including the uterus: from a mid-sagittal plane, an axial
plane of cervix can be obtained in 80 % and a coronal plane
in 20 %of the cases; in cases of uterine malformations, the
extent of the cervix and the limits of the cervical canal
may be studied better.Diagnosis of associated vaginal
anomalies can be done by trans perineal acquisition of the
pelvic floor volume after filling the vagina with gel or
saline; an axial plane can be obtained from a mid-sagittal
plane.
8. MRI- It is non-invasive and it has no radiation. It gives
a reliable and objective representation of the examining
organs in the sagittal, transverse and coronal plane (three
dimensions). It can be used for diagnosis in cases of
complex and obstructing anomalies. Electronic storage of the
diagnostic procedure is, nowadays, routinely done for
re-evaluation.
9. Hysteroscopy- It is minimally invasive giving the
additional opportunity of treating T-shaped, septate and
bicorporeal septate uterus. Its objective includes
estimation of the cervical canal and endometrial cavity
(differential diagnosis of T-shaped and infantile uterus).
It provides a minimal invasive evaluation of the vagina
and/or cervix in case of virgo. Electronic storage of the
procedure is, nowadays, routinely done for re-evaluation.
10. Endoscopy; laparoscopy and hysteroscopy- It provides
highly reliable information for the anatomical status of the
vagina (vaginoscopic approach), cervical canal, uterine
cavity, tubal ostia, external contour of the uterus and the
intra- peritoneal structures.
11. The invasiveness of the laparoscopic approach makes it
not acceptable as a first-line screening procedure; it
complements indirect imaging in the diagnosis of more
complex anomalies in combination with possible surgical
actions. It offers supplementary information about partial
or total absence of Fallopian tubes and abnormal
localisation of ovaries.
12. Highest degrees of overall diagnostic accuracy were in
de-creasing order: 3D US (97.6 %), sonohysterography (SHG;
96.5 %), 2D US (86.6) and hysterosalpingography (HSG; 86.9
%). MRI was shown to be able to correctly subclassifiy 85.8%
of anomalies. Overall, it appears that 3D US may be more
accurate than MRI in sub-classifying malformations, although
it should be noted that sub-classification is hindered due
to the subjective nature of the previous classifications
adopted.
Uterine wall thickness:
1. Uterine wall thickness is an important parameter and a
reference point for the definitions of dysmorphic T- shaped,
septate and bicorporeal uteri according to the new
classification system. The adoption of an objective
criterion for the definition of uterine deformity is one of
the advantages of the new classification system since
according to AFS classification the detection of anomalies
was based only on the subjective impression of the clinician
performing the test. Although myometrial thickness at the
various uterine regions cannot be easily assessed with
endoscopic techniques, it can be measured with ultrasound or
MRI.
2. Uterine wall thickness- This is the distance between the
line connecting the tubal ostia and the external uterine
profile obtained with 3D US, MRI and, at times, with 2D US
CONGENITAL MALFORMATIONS OF THE UTERUS
American Fertility Society has classified the
malformations (Figure 10) as:
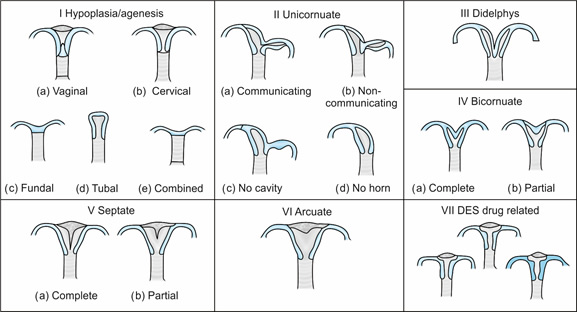
Figure 10 ASRM classification
of Mϋllerian anomalies
Class I: Agenesis or
Hypoplasia—
Segmental or Complete
Agenesis or hypoplasia may involve the vagina, cervix,
fundus, tubes or any combination of these. Mayor-Rokitansky-Kuster-Hauser
(MRKH) syndrome is the most well known example of this.
Class II: Unicornuate Uterus
with or
without Rudimentary Horn
When an associated horn is present, this class is
subdivided into communicating (continuity with main uterine
cavity documented) or non-communicating. The
noncommunicating variety is further subdivided on the basis
of whether endometrium is present or absent in the
rudimentary horn. The clinical significance of these types
is that they are invariably associated with ipsilateral
renal and ureteric agenesis.
Noncommunicating accessory horns
that have endometrial cavity are the most common unicornuate
subtype and are clinically important too. They are
associated with high morbidity and mortality. When the
accessory horn becomes obstructed, complication like
hematometra can occur. There is also a risk of developing
endometriosis.
Although normal pregnancies do
occur, this type is associated with poorest obstetrical
performance. This may be due to diminished uterine
vasculature of deficient uterine musculature. A study12 of
393 pregnancies revealed 54.2 percent had normal deliveries,
43.3 percent had preterm deliveries, 4.3% had ectopic
pregnancies and 34.4 percent had spontaneous abortions.
About 2 percent pregnancies occur in accessory horn. Hence
the noncommunicating horn should be excised prior to
pregnancy.
|
|
External contour |
Uterine cavity |
Separation of horns |
Cervix |
|
Normal |
Convex, flat or, 1 cm fundal cleft |
Convex or flat (Two corneal ostia) |
Single triangular cavity |
Single |
|
Unicornuate |
Convex or flat |
Convex or flat (single corneal ostium) |
Single banana shaped cavity |
Single |
|
Didelphys |
Two well formed uterine cornu, convex or flat |
Two well formed uterine cornu with convex fundal
contour in each with no communication |
Two horns widely divergent, at an obtuse angle |
Double |
|
Bicornuate |
Fundal cleft >1cm |
Two well formed symmetric uterine cornu with convex
fundal contour in each fused caudally, communicate |
Two horns widely divergent, at an obtuse angle |
Single or Double |
|
Septate |
Convex, flat or <1 cm fundal cleft |
Two well formed symmetric uterine cornu, communicate |
Two horns close, acute angle at the center |
Single |
|
Subseptate |
Convex, flat or <1 cm fundal cleft |
Two well formed symmetric uterine cornu, communicate |
Two horns close, acute angle at the center |
Single |
|
Arcuate |
Convex, flat or <1 cm fundal cleft |
Single cavity with a broad shallow indentation |
Obtuse angle at the center |
Single |
Class III: Didelphys Uterus
Complete or partial duplication of vagina, cervix and
uterus is a feature of this type.
About 11 percent of all uterine
abnormalities are of this type. Complete type is
characterized by two hemiuteri, two endocervical canals,
with cervices fused at the lower uterine segment. Each
hemiuterus is associated with a fallopian tube. Ovarian
malposition may also be present. The vagina may be single or
double; more commonly double (75%). Occasionally, the
vaginal septum may be transverse.
This type is associated with
renal abnormalities in 20 percent of the cases. A syndrome
called is Wunderlich-Herlyn-Werner syndrome has been
described13. This consists of obstructed unilateral vagina
with uterus Didelphys with ipsilateral ureteric and renal
agenesis.
Class IV: Bicornuate Uterus—Complete or partial
Complete bicornuate uterus is characterized by a uterine
septum that extends from the fundus to the cervical os.
Partial type has a septum which is limited to the fundus. In
both, there is a single cervix and vagina. Obstetric outcome
depends on the length of the muscular septum, i.e. whether
the bicornuate uterus is complete or partial. A partial
bicornuate uterus has a 28 percent incidence of spontaneous
abortion while in case of a complete bicornuate uterus; the
incidence of abortion is 66 percent.
Class V: Septate Uterus—Complete or partial
A single uterus has a complete or partial septum. The
septum is located in the midline fundal region. It is made
up of poorly vascularized fibro muscular tissue. There are
various variations of the septum. Sometimes a complete
septate uterus is associated with a septate vagina. A
variant septate abnormality exists which is characterized by
the triad of complete septate uterus, duplicated cervix and
septate vagina.14 It is undistinguishable from uterus
Didelphys except by doing laparoscopy or a 3D USG. On
laparoscopy, it is identified by its convex external
contour.
A rare variant of septate uterus is Robert uterus.15 This
is characterized by a complete septum and a noncommunicating
hemiuteri with a blind horn.
Patients with a septate uterus have no difficulty in
conceiving. Yet, they have poorest reproductive outcome of
all the Müllerian anomalies.
Class VI: Arcuate Uterus
A small septate indentation is seen at the fundus.
Class VII: DES Related Abnormalities
A “T” shaped uterine cavity is seen.
Defects Not Classified by the AFS
• Transverse vaginal septum
• Vaginal Atresia
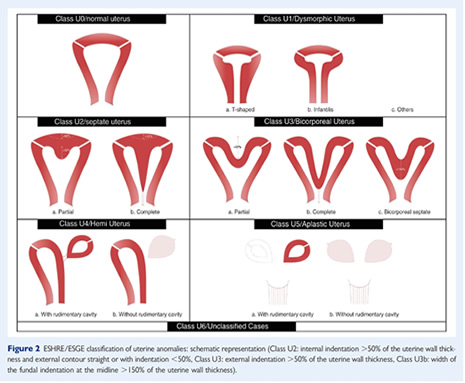
The ESHRE/ESGE classification
It was believed that the existing AFS classification had
limitations (20). There was need to have a classification
that was:
1. Clear and accurate
2. Which correlated with patient management
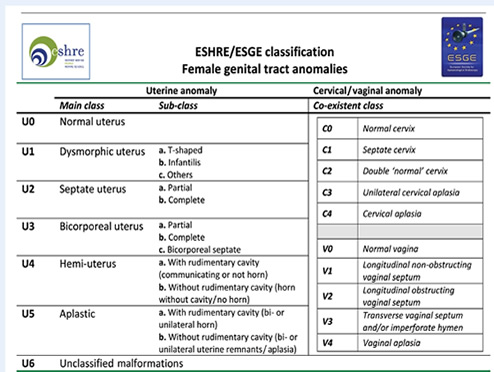
3. was simple
and friendly
MANAGEMENT
Müllerian Aplasia
This basically includes management of vaginal agenesis
or MRKH syndrome. Here, the management comprises of two
parts:
1. Creation of neovagina
2. Fertility treatment
Creation of Neovagina
Two methods have been described, nonsurgical and
surgical. The nonsurgical method basically involves use of
prosthetic moulds which deepen the existing pouch to create
a satisfactory space for coitus. While surgical methods
involve creation of neovagina, the strategy is to develop a
space between the rectum and the bladder. Various tissues
have been used to cover the newly created space. Full
thickness or part thickness skin grafting is the most
popular technique. However, skin grafting is associated with
scar formation or contractures. Alternately, human amnion,
not stripped from chorion is used as a graft material.16
More elaborate plastic surgical techniques include use of
transposition flaps and autologous buccal mucosa. Use of
artificial dermis and absorbable adhesion barrier shows
promise as exogenous graft material to be used in neovagina.
Interceed (Ethicon) has been used as an absorbable adhesion
barrier. The neovagina epithelializes within 1 to 4 months.
Some surgeons use a bowel segment in place of skin graft.
However, this is associated with troublesome leucorrhea
after the surgery.
Fertility Treatment
Since, most of these women have healthy and functioning
ovaries; surrogate pregnancy is a viable treatment modality.
Unicornuate Uterus
The indication for surgery is presence of endometrium in
the rudimentary horn. In case the rudimentary horn does not
have a functioning endometrium, then surgical intervention
is not indicated.
In case surgery is contemplated, laparoscopic
hemihysterectomy of the rudimentary horn is the procedure of
choice. The rudimentary horn is connected to the functioning
unicornuate uterus by a fibromuscular band. This band is of
importance as the uterine artery courses inferior to it. The
pedicle of the rudimentary horn is coagulated using bipolar
cautery. Tube and the rudimentary horn are removed, leaving
ipsilateral ovary which is usually healthy, in situ.
In the event of pregnancy in the rudimentary horn, same
procedure as nonpregnant uterus is followed. However, there
is a risk of increased bleeding due to pregnancy related
vascularization. Methotrexate has been used to treat the
pregnancy before surgical removal of the rudimentary horn.
Hysteroscopic endometrial ablation of the rudimentary
horn has been reported.17
Uterus Didelphys
Guidelines for surgical intervention are as follows:
1. Uterus didelphys with obstructive vaginal septum—Full
excision and marsupialization of the vaginal septum is the
treatment of choice. After the septum is excised,
laparoscopy may be indicated as these cases are associated
with endometriosis.
2. Uterus didelphys with nonobstructive vaginal
septum—surgery is indicated if the patient complains of
dyspareunia.
3. Currently, metroplasty is not indicated in cases of
nonobstructive didelphys uterus.
Bicornuate Uterus
Guidelines for surgical correction are as follows:
1. Bicornuate uterus seldom requires surgical management.18
2. Metroplasty should be reserved for those women with
repeated pregnancy losses or rarely in those in whom no
other cause for infertility is detected.
Septate Uterus
Uterine septum is associated with primary infertility,
recurrent miscarriages and preterm labor. In such cases,
hysteroscopic resection of the septum is indicated.19 The
decision to operate should be taken only for poor
reproductive performance.
ESHRE/ESGE consensus on workup of female genital
anomalies (21)
Recommended evaluation of asymptomatic women
Clinicians should, always, be attentive for the presence of
a congenital anomaly in asymptomatic women of reproductive
age during their routine examination, supplementing
gynaecological examination with a 2D US as follows:
Gynecological examination: the anatomy of the external
genitalia, the vagina and the cervix should be carefully
evaluated.
2D US: it should be done in a pre-defined and systematic
manner to increase its diagnostic accuracy. The shape and
the dimensions of the uterine cavity, the uterine wall
(anterior, posterior, lateral and fundal width) and external
uterine contour should be recorded in a systematic way in
longitudinal and transverse planes.
The absence of findings suspicious for the presence of an
anomaly should not be considered as definite and the
presence of one could not be excluded.
Positive findings should be used for documentation only
and counselling of the patients for further investigation
given that they are asymptomatic women.
Recommended evaluation of symptomatic women
Gynecological examination with careful evaluation and
recording of the external genitalia, vaginal and cervical
anatomy.
2D US (vaginal) in a pre-defined and systematic manner
(to increase its diagnostic accuracy), where the shape and
the dimensions of the uterine cavity, the uterine wall
(anterior, posterior, lateral and fundal width) and external
uterine contour should be recorded in a systematic way and
pre-defined way in longitudinal and transverse planes.
Measurements of 2D US examination should be used as a
referendum for the evaluation of uterine anatomy deviations
in 3D ultrasound.
3D US (vaginal) in a pre-defined and systematic manner
where the shape and the deviations from normal cervical and
uterine anatomy should be recorded and documented.
REFERENCES
1. Steinmetz GP. Formation of artificial vagina. West J
Surg 1940; 48: 169-73.
2. Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey
P. Clinical implications of uterine malformations and
hysteroscopic treatment result. Hum Reprod Update 2001; 7:
161-74.
3. Frank R. The formation of an artificial vagina without
operation. Am J Obstet Gynecol 1938; 35: 1053-5.
4. Stray-Pederson B, Stray-Pederson S. Etiologic factors and
subsequent reproductive performance in 195 couples with a
prior history of habitual abortion. Am J Obstet Gynecol
1984; 148: 140-46.
5. Amesse LS, Pfaff-Amesse T. Congenital anomalies of the
reproductive tract. [book auth.] Hurd WW, Falcone T.
Clinical Reproductive Medicine and Surgery. New York:
Elsiever 2000; pp 235-9.
6. Golan A, Langer R, Bukovsky I, Caspy E. Congenital
Anomalies of Müllerian System. Fertil Steril 1989; 5:
747-55.
7. Carson SA, Simpson JL, Elias S, Gerbie AB, Buttram VC Jr,
et al. Heritable aspects of uterine anomalies. Fertil Steril.
1983; 1: 86-90.
8. Turunen A, Unnerus CE. Spinal changes in patients with
congenital aplasia of the vagina. 46, 1967, Acta Obstet
Gynecol Scand 1967; 1: 99-106.
9. Willemsen WN. Combination of Mayer-Rokitansky-Kuster and
Klippel Fiel syndrome-a case report and literature review.
Eur J Obstet Gynecol Reprod Biol 1982; 4: 229-35.
10. Aughton DJ. Müllerian duct abnormalities and
galactosemia heterozygosity: report of a family. Clin
Dysmorphol 1993; 1: 55-61.
11. Erdem M, Bilgin U, Bozkurt N, et al. Comparison of
transvaginal sonography and saline infusion
sonohysterography in evaluating the endometrial cavity in
pre and post menopausal patients with abnormal uterine
bleeding. Menopause. 2007; 14: 846-52.
12. C Lin P. Reproductive outcomes in women with uterine
anomalies. J Womens Health (Larchmt), 2004; 1: 33-9.
13. Tridenti G, Bruni V, Ghirardini G. Double uterus with
blind hemivagina and ipilateral renal agenesis: clinical
variants in three adolescent women.case report and
literature review. Adolesc Pediatric Gynecol 1995;8: 20.
14. Giraldo JL, Habana A, Duleba AJ, Dokras A. Septate
Uterus associated with cervical duplicationand vaginal
septum. J Am Assoc Gynecol Laparosc, 2000; 2: 277-9.
15. Robert HG. Uterus cloisonne avec cavite borgne sans
hematometeri. CR Soc Fr Gynecol, 1989; 767-7.
16. Nisolle M, Donnez J. Vaginoplasty using amniotic
membranes in cases of vaginal agenesis or after vaginectomy.
J Gynecol Surg 1992; 1: 25-30.
17. Hucke J, DeBruyne F, Campo RL, Freikha AA. Hysteroscopic
treatment of congenital uterine malformations causing
hemihematometra: a report of three cases. Fertil Steril, 58:
823-57.
18. Jones HW. Reconstruction of congenital uterovaginal
anomalies. [book auth.] Murphy AA, Jones HW Rock JA. Female
Reproductive Surgery. Baltimore: Lippincott Williams and
Wilkins 1992, 246.
19. Valdes C, Malini S, Malinak LR. Ultrasound evaluation of
female genital tract abnormalities. Am J Obstet Gynecol,
1984; 149: 285-92.
20. Grimbizis, G. F., Gordts, S., Di Spiezio Sardo, A.,
Brucker, S., De Angelis, C., Gergolet, M., … Campo, R.
(2013). The ESHRE-ESGE consensus on the classification of
female genital tract congenital anomalies. Gynecological
Surgery, 10(3), 199–212. http://doi.org/10.1007/s10397-013-0800-x
21. Grimbizis, G. F., Di Spiezio Sardo, A., Saravelos, S.
H., Gordts, S., Exacoustos, C., Van Schoubroeck, D., …
Campo, R. (2016). The Thessaloniki ESHRE/ESGE consensus on
diagnosis of female genital anomalies. Gynecological
Surgery, 13(1), 1–16. http://doi.org/10.1007/s10397-015-0909-1 |











