|
ACQUIRED COAGULATION
DISORDERS IN By: • DR. PANKAJ DESAI • DR. N. PALANIAPPPAN
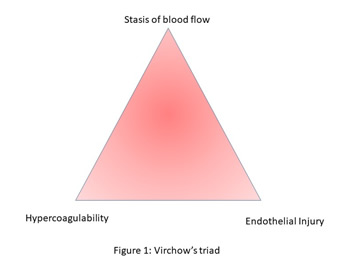
Rudolf Virchow (1) in 1856 postulated
the prerequisite triad that invites venous thrombosis as This is shown in Figure 1 Figure 1 be inserted here Any mild risk factor which triggers one
of these three along with pregnancy (which in itself is a
risk factor) can lead to venous thrombosis or a coagulation
disorder. This chapter, in particular, will deal with only
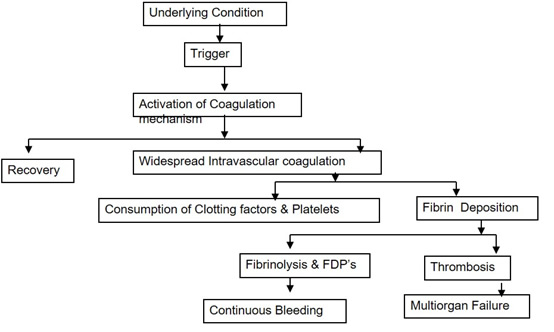
acquired coagulation disorders like DIC - Disseminated Intra vascular Coagulation Disseminated Intravascular Coagulation (DIC) is the rapid activation of intravascular coagulation leading to the deposition of fibrin within the circulatory system. Consumption of coagulation factors would lead to bleeding diathesis, although a minor percentage of individuals may go on to develop widespread thrombosis with peripheral organ ischemia. Usually, the greater risk of coagulopathy comes from the consumption of clotting factors and platelets secondary to massive hemorrhage. DIC may arise from various situations in obstetrics but is usually a secondary phenomenon to a “trigger” of coagulation activity. It would be a prudent move to identify these trigger factors, anticipate DIC and act wisely in the beginning. A failure to anticipate DIC is cited as a major deficiency in the care of women who die from obstetric hemorrhage. Trigger Mechanisms A. Vascular Endothelial Injury Once DIC sets in, there is a potential
for a vicious cycle, with further consumption of clotting
factors and platelets and bleeding justifying the
terminology of “consumption coagulopathy” as shown in fig 2 Figure 2 be inserted here Risk Factors Placental Abruption This remains the commonest cause of coagulation failure in obstetrics and is related directly to the degree of placental separation and hypovolemic shock. In severe placental abruption with a dead fetus, profound hypofibrinogenemia has been reported in about one-third of cases but is much less common if the fetus is alive (2 3 ). This initial mechanism is due to the release of thromboplastins, but in severe abruption hypovolemic shock, large volume transfusion and high levels of fibrin degradation products (FDP’s) that act as anticoagulants themselves will accentuate the situation. Accidental hemorrhage is now accepted as an obstetric vasculopathy. As a result, the pathophysiology involved in the process of accidental hemorrhage has to be at the fetomaternal interface. The anchoring cytotrophoblasts are responsible to affix the placenta within the uterus. The entire process occurs following implantation at that point in the uterus where the implantation window opens at the time of arrival of the zygote in the uterine cavity. Following a complex process where intricate immunology and apoptosis get involved, the placenta affixes itself and remains so until that time as the delivery of the newborn occurs. Following some strange and hitherto unknown signals, once the baby is born, the placenta senses that its role is over and it readily separates from the uterus and is expelled from the uterine cavity. In accidental hemorrhage however, this process of separation and expulsion of the placenta occurs much earlier even before the baby is born. Like any premature process in nature, this can lead to devastating complications (4). Amniotic Fluid Embolism This may lead to maternal death as a result of severe pulmonary hypertension following embolization of the pulmonary vessels by fetal squams. But if the mother survives this acute event there may be an anaphylactoid reaction to the presence of the fetal tissues in the maternal circulation, pulmonary edema, and the development of an intractable bleeding diathesis due to severe DIC. In most cases, maternal death is unpredictable and unavoidable. The coagulopathy that is part of the AFE syndrome ranges from minor disturbances in laboratory coagulation studies to severe DIC (5). Any or all of the following hematologic laboratory abnormalities may be present: elevated fibrin split products of D-dimer products, decreased fibrinogen, thrombocytopenia, and prolonged partial thromboplastin and prothrombin times. The exact incidence of coagulopathy with AFE is unknown, but it is common among those who survive the initial event. On rare occasions, it is the only manifestation present. The incidence of coagulopathy in the analysis of the national registry was 83% (6). Retained Dead Fetus The release of thromboplastic substances from the dead fetus into the maternal circulation is thought to be the trigger for DIC. Approximately 80% of patients with retained dead fetus will go into spontaneous labor within 3 weeks, but 30% of patients who remain undelivered for more than 4 weeks will develop DIC, usually a mild degree (7). Preeclampsia Preeclampsia is associated with endothelial perturbation currently thought to be due to oxidative stress and the release of reactive oxygen species by the Ischemic placenta. Sepsis Endotoxic shock can be associated with chorioamnionitis, septic abortion or postpartum sepsis. The bacterial endotoxin produces severe endothelial damage leading to fibrin deposition & DIC Management Options Goals of Management Aims of the management of DIC are as
follows: Relevant blood investigations are critical for deciding the management and prognosis of a subject with DIC. These have been tabulated in Table 1. Table 1 be inserted here Fluid Replacement in Coagulation Failure The impending necessity in the initial
stages is to maintain circulatory volume and tissue
perfusion, and resuscitation with a crystalloid and a
colloid solution should be undertaken as early as possible.
Dextran solutions should not be used because they interfere
with platelet function and can aggravate bleeding and DIC,
as well as invalidate the laboratory investigations (9). Replacement of Blood Products Fresh frozen plasma (FFP) and stored
red blood cells provide all the needed components. The use
of fresh whole blood should not be encouraged, as it cannot
be screened for possible infections. It is occasionally
necessary to give extra fibrinogen in the form of
cryoprecipitate, although sufficient amounts are usually
there in FFP, which also contains factors V VIII, and
antithrombin III. Platelets are not found in FFP, and their
functional activity rapidly deteriorates in stored blood.
The platelet count reflects both the degree of DIC and the
response to transfused blood. If there is persistent
bleeding and the platelet count is less than 50,000/ cu. mm,
the patient may be given concentrated platelets, but these
are not usually necessary to gain hemostasis (₿). This is
tabulated in Table 2. Table: 2 be inserted here Other treatment Options Heparin Heparin therapy has been used often, but there is no evidence to suggest that its use confers any benefits over supportive therapy. Heparin is contraindicated if there is hypovolemia and obviously this would include that secondary to abruptio placenta (10). Also, in a comprehensive review paper treatment with heparin is recommended in those with the non-symptomatic type of DIC (11). Activated Protein C In the case of sepsis, recombinant protein C confers advantages in terms of prevailing fibrin deposition and stimulating the immune responses (10). It has to be started within 24 hours of the onset of first organ dysfunction and is not routinely indicated in DIC in the absence of sepsis. Its use has been restricted due to its exorbitant cost. Activated protein C inhibits the generation of thrombin by inactivating factor Va and factor VIII (12, 13). Treatment with this agent decreased inflammation, as indicated by a decrease in interleukin – 6 levels, a finding consistent with the known anti-inflammatory activity of activated protein C. Furthermore, this agent has direct anti-inflammatory properties, including the inhibition of neutrophil activation, the production of cytokines by lipopolysaccharide challenged monocytes, and E selectin-mediated adhesion of cells to vascular endothelium. Activated Protein C is given as an intravenous infusion at a dose of 24 micrograms per kg body weight per hour for 96 hours Recombinant Factor VII-a Recombinant activated factor VII (rFVIIa) is a recombinant form of the naturally occurring protease. Since 1998, rFVIIa has been approved and used extensively for the control of bleeding or surgical prophylaxis in patients with hemophilia who have inhibitors to coagulation factors (14). rFVIIa has been approved for use only in Glanzmann thrombasthenia and factor VII deficiency. Other than in these indications, any other use is considered as “off label” use. Owing to the cost of this novel drug its use needs to be well justified before prescription. Existing literature does not support its routine use. It is to be given to patients with PPH only as a last resort after routine medical and surgical therapies have been done just before a hysterectomy. It is given as a 90ug/kg as a single bolus over 3-5 min. Check after 20minutes for temperature, academia, serum calcium, platelet and, fibrinogen. If no improvement administer a second dose of 90ug/kg (15). Thrombocytopenia Thrombocytopenia complicates up to 10% of all pregnancies and may result from a number of causes (Table 3). Some of these are unique to pregnancy, while others may occur with increased frequency during gestation and still others bear no relationship to pregnancy per se. While some thrombocytopenic disorders are not associated with adverse pregnancy outcomes, others are associated with significant maternal and/or neonatal morbidity and mortality (16). Table 3 be inserted here Gestational “incidental” Thrombocytopenia Over 75% of pregnant women noted to have low platelet
counts have no apparent predisposing factors. Such isolated
maternal thrombocytopenia is usually mild and occurs during
the latter half of pregnancy. There is no adverse
consequence to the mother or neonate. However, extreme care
is required in ruling out occult preeclampsia, HELLP
syndrome as well as Idiopathic Thrombocytic Purpura. Such
patients should be subjected to assessment of liver function
tests (SGOT, SGPT, S. bilirubin) and test for ongoing
hemolysis (hemoglobin, LDH, reticulocyte count and RBC
morphology for microangiopathic hemolysis). One should also
do tests for anti-platelet antibodies, Lupus Anticoagulant,
Anticardiolipin Antibody, and Antinuclear Factor. Such
patients do not require a cesarean section for this
indication. The newborn does not develop any
thrombocytopenia at birth or early neonatal life. Thrombotic thrombocytopenic purpura (TTP) and hemolytic-uremic syndrome (HUS) are two important acute conditions to diagnose. Clinically, a number of conditions present with microangiopathic hemolytic anemia and thrombocytopenia, including cancer, infection, transplantation, drug use, autoimmune disease, and pre-eclampsia and hemolysis, elevated liver enzymes and low platelet count syndrome in pregnancy. Despite overlapping clinical presentations, TTP and HUS have distinct pathophysiologies and treatment pathways. TTP consists of a pentad of: a) Microangiopathic hemolytic anemia Such classical pentad is seen in 40% of patients only (17). It mostly occurs before delivery and 60% of cases occur before 24th week of gestation. It is difficult to differentiate TTP from preeclampsia especially during 3rd trimester. However, depressed serum antithrombin III suggests preeclampsia. Management includes plasma exchange with 80% success. Relapses and recurrences are common (18). HUS usually starts as acute renal failure and is more common in the peripartum period. Microangiopathic hemolytic anemia is extremely common. Liver functions are not compromised. Mild thrombocytopenia is common. Many affected patients develop hypertension and acute renal failure while 20% of patients of HUS ultimately go on to develop chronic renal failure. Dialysis is indicated. Efficacy of plasma exchange is not as certain as TTP. Auto Immune Thrombocytopenic Purpura: Immune thrombocytopenia (ITP) occurs in one or two of every 1,000 pregnancies [19] and accounts for 5% of cases of pregnancy-associated thrombocytopenia. Despite its rarity compared to gestational thrombocytopenia (vide infra), ITP is the most common cause of isolated thrombocytopenia in the first and early second trimesters [19 20, 21]. The pathophysiology of ITP has been classically believed to reflect the accelerated clearance of platelets coated by IgG anti-platelet autoantibodies. These cross over the fetal compartment and lead to neonatal thrombocytopenia and even hemorrhage. In 10% of adults, ITP runs a chronic course with relapse and remissions. As in the non-pregnant state, the diagnosis of ITP is a clinical diagnosis of exclusion. The likelihood that a patient suffers from ITP rather than incidental thrombocytopenia of pregnancy (vide infra) increases as the platelet count decreases; however, no specific platelet count below which incidental thrombocytopenia may be excluded has been defined. Furthermore, since many patients with apparent incidental thrombocytopenia have elevated levels of platelet-associated IgG, platelet antibody tests do not differentiate these syndromes [22]. Patients may be asymptomatic and hence diagnosis is often first suspected during laboratory evaluations. The laboratory tests to detect anti-platelet antibody are not often available and quite laborious. Depending on the methodology used, they could also be non-specific or insensitive. Hence, the diagnosis of auto-immune thrombocytopenia is often based on excluding other medical and obstetric conditions both by clinical methods and investigations. High MPV (Mean Platelet Volume), presence of megathrombocytes in the smear, increased or adequate megakaryocytes in the marrow aspirate, normal results of other blood counts and coagulation studies, lack of splenomegaly, normal hepatic and renal function and lack of clinical or laboratory evidence of SLE and antiphospholipid antibody syndrome are all essential to make a diagnosis of autoimmune thrombocytopenic purpura. History of recent exposure to drugs and viral as well as other infections (including HIV) known to produce thrombocytopenia is also important. The patient may be a known case of ITP who becomes pregnant or ITP may be first time suspected and diagnosed in a woman who is already pregnant Pregnancy does not exacerbate the course and severity of ITP. However, the disease adversely affects maternal and fetal outcomes. It is important to avoid maternal morbidity and mortality both during pregnancy and delivery and at the same time one also has to be careful in preventing complications related to thrombocytopenia in the newborn. The clinical management of pregnancy is a complex job. It obviously requires close collaboration between the obstetrician and hematologist. Pregnant women with ITP require careful monitoring. The protocol needs that she should be seen monthly in the first and second trimester, every 2 weeks after 28 weeks, and weekly after 36 weeks. Decisions concerning the need for therapy are determined chiefly by the patient’s symptoms. Most important is whether active bleeding is present. However, the absolute platelet count should be considered as term approaches. There is no evidence to support the opinion that platelet counts should be kept higher in the asymptomatic pregnant woman than in other thrombocytopenic patients (Ĵ). More aggressive treatment is recommended later in pregnancy to prepare the patient for labor and delivery (16). Patients who are symptomatic and those with counts below 50,000/cu mm are treated with oral steroids with an initial dose of 1mg/kg/day. Majority of patients would improve within 4 weeks. Others would need intravenous high dose immunoglobulin at a dose between 400mg/kg/day x 5 days and 1gm/kg/day x 2 days. Over 80% of patients respond to this treatment by 5th or 6th day. For unresponsive patients, splenectomy is a responsible alternative during the second trimester of pregnancy. There is very little experience with either of the thrombopoietic agents in pregnancy, and both are considered category C for this indication. A pregnancy registry has been developed for patients who become pregnant while taking either Eltrombopag or Romiplostim. Likewise, it is not known whether either of these agents is excreted in human milk, and thus their safety in nursing mothers has not been established (16). In managing the delivery of the pregnant patient with ITP, some unique issues must be considered. In terms of maternal management, the primary consideration is achieving a platelet count sufficient to minimize maternal hemorrhage not only during vaginal delivery but in case of cesarean section. Epidural anesthesia is also commonly used during parturition, and adequate hemostasis is required to minimize the risk of any resulting neurologic complications that might arise. The American Society of Hematology guidelines (Table 4) suggests that a maternal platelet count of 50,000/ μl is sufficient for a vaginal delivery as well as cesarean section. The BCSH guidelines recommend that a platelet count of 80,000/μl be attained for cesarean delivery as well as for epidural anesthesia, based on a retrospective review in which epidural anesthesia was successfully delivered with no neurologic complications in 30 thrombocytopenic women with platelet counts between 69,000-98,000/μl (23). Thus, though no prospective, randomized data is available to address this issue definitively, most experts consider a platelet count in the range of 80,000/μl adequate for epidural anesthesia and either vaginal delivery or cesarean section in the parturient. Since this may be significantly higher than the therapeutic platelet count range targeted earlier in pregnancy, additional therapy may be required in some pregnant patients as term approaches. Table 4 be inserted here Majority of mothers with ITP with a negative
anti-platelet antibody have a negligible risk of severe
neonatal thrombocytopenia. Once again, problems related to
platelet antibody determination have to be remembered.
Recent literature suggests that in the rarity of neonatal
complications, caesarian delivery should be curtailed
(Samuels et al). Morbidity and mortality in the newborns
occur equally irrespective of vaginal and caesarian births.
One may summarize the subject as follows. Pregnancy and SLE occur in the same age group. 20% of
patients with SLE have thrombocytopenia. Another 20% have
associated antiphospholipid antibody syndrome. Acquired hemophilia Acquired hemophilia is a rare condition where an antibody is directed against factor VIII. It is an autoimmune disease where the precipitating factor remains unknown. These are usually IgG in nature. However, interestingly despite the passage of antibodies into the fetus which would decrease in neonatal factor VIII level, hemorrhagic problems in the newborn are unusual. The most likely explanation for the formation of these antibodies is related to a temporary breakdown in the mother’s tolerance to her own factor VIII. Its onset, duration, and severity are variable. Majority of patients present within 3 months of delivery with severe bleeding, extensive bruising, bleeding from GI tract and genitourinary tract. Hemarthrosis is uncommon. The diagnosis is based on normal platelet count, normal prothrombin time, markedly prolonged partial thromboplastin time which is not corrected by the addition of normal plasma. The potency of the antibody is determined which could reflect the severity of the problem. Management of bleeding episodes in this situation is
difficult as a conventional amount of Factor VIII is not
only ineffective but also enhances antibody formation.
Immunosuppressive agents together with corticosteroids,
intravenous gammaglobulin shows variable results Drugs producing thrombocytopenia Many drugs can produce thrombocytopenia. A list containing important and common drugs is given in table 5 Table 5 be inserted here References: 1. April Wang Armstrong; David E. Golan; Armen H.
Tashjian; Ehrin Armstrong (2008). Principles of
pharmacology: the pathophysiologic basis of drug therapy.
Philadelphia: Wolters Kluwer Health/Lippincott Williams &
Wilkins. p. 396 Table 1
Table: 2 Blood and components for consumptive Coagulopathy
Table 3
Abbreviations: ITP, immune thrombocytopenia; HIT, heparin induced thrombocytopenia; HUS, haemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura; HBV, hepatitis B virus; EBV, Epstein – Barr virus; CMV, cytomegalovirus. Table 4 Management of Delivery in Patients with Pregnancy Associated ITP – ASH and BCSH Guidelines
Abbreviations: ITP, immune thrombocytopenia; ASH, American Society of Hematology; BSCH, British Committee for Standards in Haematology; IVIg, intravenous immune-globulin.
FIGURE 1
Figure 2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||