| |
|
|
RECURRENT PREGNANCY LOSS
By:
• DR. PANKAJ DESAI
Dean (Students) and Assoc.
Professor [VRS]
Dept. Of Obstetrics & Gynecology
Medical College & S. S. G. Hospital
BARODA
• DR. MONA SHROFF
Fmr. Asst. Professor
Dept. Of Obstetrics & Gynecology
Surat Municipal Institute of Medical Education and Research
SURAT
INTRODUCTION:
A pregnancy loss (miscarriage) is defined as the
spontaneous demise of a pregnancy before the fetus reaches
viability. The term therefore includes all pregnancy losses
from the time of conception until 24 weeks of gestation (20
weeks in few countries with advanced neonatal care
infrastructure).Majority of these sporadic losses are due to
random numeric chromosomal errors.
Recurrent pregnancy loss (RPL) is one of the most
emotionally traumatic, disconcerting and challenging areas
in reproductive medicine because the etiology is often
unidentified and research on the etiology, evaluation, and
management of RPL is often erroneous. Typical methodological
shortcomings include failure to adhere to generally accepted
criteria for RPL, improper selection of controls,
ascertainment bias ,disparate monitoring of cohorts, no
exclusion of aneuploid fetuses, lack of stratification for
important factors such as number of previous losses,
premature termination of study after interim analysis, and
excessive post-randomization patient withdrawal [1].
DEFINITION:
The definition of RPL is varied which makes research on
evaluation, management and counselling more challenging.
●Two or more failed clinical pregnancies as documented by
ultrasonography or histopathologic examination [2].
●Three consecutive pregnancy losses, which are not required
to be intrauterine [3-6].
Non-visualized pregnancy losses (biochemical pregnancy
losses and/or pregnancies of unknown location) had the same
negative impact on future live birth as an intrauterine
pregnancy losses. These definitions also do not take into
account the effect of maternal age or the gestational age at
which the miscarriage occurred.
RPL can be further divided into primary or secondary.
Primary RPL refers to pregnancy loss in women who have never
carried to viability. Secondary RPL refers to pregnancy loss
in a woman who has had a previous live birth. The prognosis
for successful pregnancy is better with secondary RPL [7, 8]
Further classification can be Pre-embryonic (<4 weeks),
Embryonic (5-9 weeks), and Fetal (>10 weeks)
INCIDENCE:
Approximately 15 percent of pregnant women experience
sporadic loss of a clinically recognized pregnancy. Just 2
percent of pregnant women experience two consecutive
pregnancy losses and only 0.4 to 1 percent have three
consecutive pregnancy losses [9). At very early gestational
ages (e.g., at less than 6 weeks of gestation) the risk of
miscarriage is 22 to 57 percent versus 15 percent at 6 to 10
weeks and 2 to 3 percent after 10 weeks [10, 11]). The
prevalence of miscarriage is higher with increasing maternal
age, most likely due to poor oocyte quality (12)
TABLE I: Age and chances of Miscarriage
|
Age(Yrs.) |
Spontaneous Miscarriage risk (%) |
|
Overall |
11 % |
|
20-30 yrs. |
9-17% |
|
35 yrs. |
20% |
|
40 yrs. |
40% |
|
45 yrs. |
80% |
RISK FACTORS AND ETIOLOGY:
RPL is a heterogeneous condition, with numerous causes,
diverse treatment options and enormous psychological
implications. It is multidisciplinary, involving gynecology,
genetics, endocrinology, immunology, pediatrics and internal
medicine.
Two major concerns for the physician and the couple are:
the cause and the risk of recurrence. The etiology can be
identified in less than 50 percent of patients. General
etiological categories of RPL include anatomic,
immunological, genetic, endocrine, infectious, thrombophilic,
male factors and environmental factors. Prognosis is based
on the number of preceding pregnancy losses and female age.
Previous pregnancy loss — (13)
Table II: Number of previous Losses and risk of
miscarriage
|
Number of previous losses |
Risk of miscarriage (%) |
|
Zero |
11-13 |
|
1 |
14-21 |
|
2 |
24-29 |
|
3 |
31-33 |
Genetic factors
• Parental chromosomal rearrangements [14, 15] in 2–5%
of couples with recurrent miscarriage, one of the partners
carries a balanced structural chromosomal anomaly, most
commonly a balanced reciprocal or Robertsonian translocation
less commonly, an inversion. Although carriers of a balanced
translocation are usually phenotypically normal, their
pregnancies are at increased risk of miscarriage or
congenital malformations and/or mental disability secondary
to an unbalanced chromosomal arrangement. The risk of
miscarriage is influenced by the size and the genetic
content of the rearranged chromosomal segments. Balanced
translocations are more common in the females likely to
result in pregnancy loss if the translocation is of maternal
origin.
• Chromosomal abnormalities of the embryo [16-17].
— In couples with RPL aneuploidies account for 30–57% of
further miscarriages. The risk of aneuploidy and association
with the number of previous miscarriages is contradictory in
several studies but mostly as the number of miscarriages
increases, the risk of euploid pregnancy loss increases. The
relationship between the karyotype of the abortus and risk
of RPL requires further study to better define which
abnormalities are likely to be recurrent. Recurrent
aneuploid losses may be associated, in part, with the older
age of the mothers.
The likelihood that RPL is related to parental karyotypic
abnormality is higher when:
• Young maternal age at second miscarriage.
• History of three or more miscarriages.
• History of two or more miscarriages in a sibling or the
parents of either partner
• A family history of stillbirth or an abnormal live--born.
Immunologic factors:
Antiphospholipid syndrome (APS) (18, 19) — several
autoimmune diseases have been linked to poor obstetric
outcome, but APS is the only immune condition in which
pregnancy loss is a diagnostic criteria for the disease.
Five to 15 percent of patients with RPL may have APS
compared to 2% in normal obstetric patients. APS is the most
important treatable cause of recurrent miscarriage
The mechanisms by which antiphospholipid antibodies cause
pregnancy morbidity include inhibition of trophoblastic
function and differentiation, activation of complement
pathways at the maternal–fetal interface resulting in a
local inflammatory response and thrombosis of the
uteroplacental vasculature.
Other immunological factors (20-23) — allogeneic factors
may cause RPL by a mechanism similar to that of graft
rejection in transplant recipients. If the blastocyst is
developmentally normal and intact, the embryo is entirely
protected by trophoblast cells. In some pregnancies, the
blastocyst is genetically deformed and not fully intact so
paternally-derived antigens are exposed to the maternal
immune system, leading to a rejection response. A secondary
immune response would be expected to cause early rejection
in cases of RPL.
Alternatively, some mothers with RPL may lack essential
components of the networks that provide immunological
protection to the embryos, such as appropriate expression of
complement regulatory proteins. Deregulation of the normal
immune mechanism, although not well defined, probably
operates at the maternal-fetal interface and may involve
increased activity of uterine natural killer (uNK) cells,
which appear to regulate placental and trophoblastic growth,
local immunomodulation, and control of trophoblastic
invasion.
Thrombophilia and fibrinolytic factors[24-25] —
Thrombosis of spiral arteries and the intervillous space on
the maternal side of the placenta can impair adequate
placental perfusion leading to late fetal loss, FGR,
placental abruption, or preeclampsia. A relationship to
early pregnancy loss is conflicting and may be restricted to
specific thrombophilic defects that have not been fully
defined, or the presence of multiple defects. A systematic
review of the association between fibrinolytic defects and
RPL found a significant association for factor XII
deficiency.
Procoagulant microparticles can also contribute to the
hypercoagulable state and likely to interfere with
successful implantation and fetal growth. These were shown
to be associated with early and late unexplained pregnancy
loss.
Uterine factors [26-33]. — Acquired and congenital
uterine abnormalities are responsible for 10 to 50 percent
of RPL.
Anomalies — Congenital uterine anomalies are
present in 10 to 15 percent of women with RPL versus 7
percent of all women but there is a wide variability in
diagnosis and inclusion criteria (first/second trimester)
Pregnancy loss may be related to impaired uterine distention
or abnormal implantation due to decreased vascularity in a
septum, increased inflammation, or reduction in sensitivity
to steroid hormones with possibly coexisting cervical
weakness. The septate uterus is the most common uterine
abnormality associated with RPL and the poorest reproductive
outcome due mechanisms not clearly understood. The
miscarriage rate in women with untreated septum in small
observational studies is greater than 60 percent. The longer
the septum, the worse the prognosis possibly due to poor
blood supply and implantation. Arcuate uteri are more
associated with second trimester losses.
Leiomyoma — Submucous leiomyomas that protrude
into the endometrial cavity can impede normal implantation
as a result of their position, poor endometrial receptivity
of the decidua overlying the myoma, or degeneration with
increasing cytokine production but no clear association has
been proven.
Endometrial polyps — there have been no data
showing a relationship between endometrial polyps and RPL.
Intrauterine adhesions — intrauterine adhesions
or synechiae lead to pregnancy loss because there is
insufficient endometrium to support fetoplacental growth.
The main cause of intrauterine adhesions is intrauterine
interventions traumatizing the basalis layer, leading to
menstrual irregularities (hypomenorrhea, amenorrhea), cyclic
pelvic pain, infertility, and RPL.
Cervical insufficiency — cervical insufficiency
could lead to recurrent midtrimester, but not early
pregnancy loss. True incidence remains unknown due to
essentially a clinical diagnosis and no specific
inter-pregnancy test
Defective endometrial receptivity — Estrogen and
progesterone prepare the endometrium for pregnancy. Normal
endometrial receptivity allows embryo attachment,
implantation, invasion, and development of the placenta.
These processes are likely to be disturbed when endometrial
receptivity is defective, resulting in unexplained
infertility and RPL. Causes of defective endometrial
receptivity and biomarkers for evaluation of endometrial
receptivity are under research. RPL may be associated with
primary receptor defect, uterine stem cell deficiency and
enhanced cellular senescence, which then results in abnormal
endometrial preparation leading to RPL.
Environmental chemicals and stress (21, 34)—There
is no high-quality evidence showing a relationship between
RPL and occupational factors, stress, or low level exposure
to most environmental chemicals. Chemicals that have been
associated with sporadic spontaneous pregnancy loss include
anesthetic gases, arsenic, aniline dyes, benzene, ethylene
oxide, formaldehyde, pesticides, lead, mercury, and cadmium.
Other
Personal habits [1, 35] — the association between RPL
and obesity, smoking, alcohol use, and caffeine consumption
is unclear. These factors may act in a dose-dependent
fashion or synergistically to increase the rate of sporadic
pregnancy loss.
Male factor [36-38]. — There is a trend toward
repeated miscarriages in women whose male partner has
abnormal sperms (e.g., poor morphology, sperm chromosome
aneuploidy, high DFI) advanced paternal age may be a risk
factor for miscarriage.
Infection[39-41] — Some infections, such as
Toxoplasma gondii, cytomegalovirus, and primary genital
herpes, are known to cause sporadic pregnancy loss, but no
infectious agent has been proven to cause RPL. The presence
of bacterial vaginosis in the first trimester of pregnancy
has been reported as a risk factor for second-trimester
miscarriage and preterm delivery, but the evidence for an
association with first trimester miscarriage is
inconsistent.
Decreased ovarian reserve[42] — Women with
unexplained RPL have a higher incidence of abnormal ovarian
reserve tests, than women with a known cause of RPL Women
with RPL and elevated day 3 FSH or low AMH may have poor
quality oocytes that fail to develop after fertilization.
Future research [43-45] — A meta-analysis of
studies evaluating whether there is an association between
cytokine polymorphisms and RPL concluded there was no more
than a mild non-significant association. Progesterone
receptor gene polymorphisms, as well as other gene
polymorphisms, may play a role in RPL.
HISTORY AND PHYSICAL EXAMINATION:
The minimum diagnostic workup of couples with RPL
consists of a complete medical, surgical, genetic, and
family history and a physical examination.
History — the history should include
• The gestational age and characteristics (e.g., anembryonic
pregnancy, live embryo) of all previous pregnancies.
Gestational age is important because RPL typically occurs at
a similar gestational age in consecutive pregnancies and the
most common causes of RPL vary by trimester. E.g.
Miscarriages related to chromosomal and endocrine defects
tends to occur earlier in gestation than losses due to
anatomic or immunological abnormalities; however, there is
significant overlap.
• Abnormalities in menstrual cycle length may be due to
endocrine dysfunction. Presence of galactorrhea, which also
suggests endocrine dysfunction (hyperprolactinemia)
• Does the family history display patterns of disease
consistent with a strong genetic influence? Is consanguinity
present?
• Was embryonic/fetal cardiac activity ever detected? RPL
prior to detection of embryonic cardiac activity also
suggests a chromosomal abnormality
• Is there exposure to environmental toxins, which may be
lethal to developing embryos?
• Is there a personal/family history of venous or arterial
thrombosis suggestive of antiphospholipid syndrome?
• History of uterine instrumentation, which may have caused
intrauterine adhesions.
• What information is available from previous laboratory,
pathology, and imaging studies?
Physical examination — Physical assessment should
include signs of endocrinopathy (e.g., hirsutism,
galactorrhea) and pelvic organ abnormalities (e.g., uterine
malformation, cervical laceration).
Mental health evaluation — screening for severe stress and
depression should be an integral part of the RPL work-up.
EVALUATION:
Parental Karyotype [16, 21, 46] — Karyotyping of couples is
part of the evaluation of RPL, despite the low yield of
abnormality, cost, and limited prognostic value. The purpose
is to detect balanced reciprocal or Robertsonian
translocations or mosaicism that could be passed to the
fetus unbalanced.
Karyotype of the abortus or products of conception [16,
47] -- Chromosomal abnormalities detectable in parental
peripheral blood preparations are an indirect and limited
indicator of fetal karyotype vis-a-vis the fetal karyotype.
• Knowledge of the karyotype of the products of
conception allows an informed prognosis for a future
pregnancy outcome
• To differentiate whether it was sporadic due to abnormal
embryo or treatment failure per se and need for further
evaluation
• A normal karyotype suggests (but does not prove) a
maternal factor as the cause of pregnancy loss, while an
abnormal karyotype is usually a sufficient explanation for a
nonviable pregnancy
• If the karyotype of the miscarried pregnancy is
abnormal(Aneuploidy), there is a better prognosis for the
next pregnancy(except unbalanced translocations)
Pitfalls of conventional POC karyotype are: - [48-50].
• Failure to cultivate: Cells from chromosomally abnormal
abortuses, are less likely to grow in culture, thereby
skewing the results of cohort studies maternal tissue
contamination. Array comparative genomic hybridization (aCGH)
does not require dividing cells, and therefore can be useful
in fetal demise with culture failure.
• Failure to seek other coexisting causes if cytogenetic
study reveals chromosomal abnormality.
• Occurrence of non-cytogenetic embryonal abnormalities.
• Type of laboratory and analysis: In some cases, karyotype
analysis of the abortus indicates a normal chromosomal
pattern, but more detailed Acgh demonstrates major
abnormalities.
• Need for surgical evacuation.
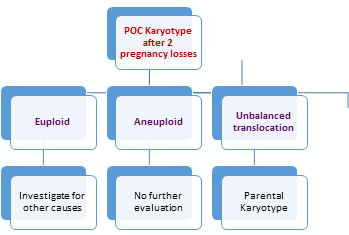
Fig I: Flow Chart for
Evaluation of Chromosomal Anomalies in RPL
Uterine assessment [51-57].— Anatomic causes of
RPL are usually diagnosed using hysterosalpingography (HSG)
, sonohysterography.(SHG) or 3D ultrasound(USG) .3D USG and
SHG are more accurate than HSG in delineating internal
contours along with outer. Ultrasound (especially 3D) is
useful for making the diagnosis of a septate/arcuate/bicornuate
uterus ,renal abnormalities associated, the presence and
location of uterine myomas and, in pregnancy, the
possibility of cervical insufficiency and assessment of
fetal viability.
Hysteroscopy, laparoscopy, or magnetic resonance imaging
(MRI) due to invasiveness and /or cost, are used as
second-line tests when additional information or therapeutic
intervention is required.
Anticardiolipin antibodies and lupus anticoagulant
[58-60]— The minimum immunology work-up for women with
RPL is measurement of anticardiolipin antibody (IgG and IgM)
and lupus anticoagulant, done twice, six to eight weeks
apart, because a low to mid positive level can be due to
viral illness and revert to normal. The anticardiolipin
antibody titre is considered elevated if medium or high
titres of both IgG and IgM isotypes are present in blood.
The detection of the lupus anticoagulant is generally based
upon an activated partial thromboplastin time, kaolin plasma
clotting time, or dilute Russell viper venom test time
Table III: Clinical and Laboratory Findings in
Reproductive Autoimmune Syndrome
|
|
Antiphospholipid Syndrome (APS) |
Reproductive Autoimmune Failure Syndrome (RAFS) |
|
Clinical features |
·
Thrombosis (≥1 unexplained
venous or arterial thrombosis, including stroke) |
·
FGR(<34 weeks) |
|
·
Autoimmune thrombocytopenia |
·
Severe Preeclampsia |
|
·
Adverse Pregnancy outcome |
·
Obstetric complications
(abruption placenta, chorea gravidarum, HELLP
syndrome) |
|
|
·
Unexplained infertility |
|
o
Three or more consecutive miscarriages before 10
weeks of gestation |
·
Endometriosis |
|
o
One or more morphologically normal fetal losses
after the 10th week of gestation |
·
Recurrent pregnancy loss |
|
o
One or more preterm births before the 34th week of
gestation owing to placental disease. |
o
≥1 consecutive and otherwise unexplained fetal
deaths (≥10 weeks) |
|
|
o
≥3 consecutive and otherwise unexplained
preembryonic or embryonic pregnancy losses |
Thyroid function [61-63] — Thyroid function should
be assessed if positive history or symptoms. Screening
asymptomatic women for subclinical thyroid dysfunction is
controversial but recommended since there is evidence of an
increased risk of miscarriage in women with subclinical
hypothyroidism and in euthyroid women with thyroid
peroxidase (TPO) antibodies.
Hypercoagulable state —Evaluation for an inherited
thrombophilia can be considered in rare cases of recurrent,
unexplained late fetal loss (after nine weeks of gestation)
associated with evidence of placental ischemia and
infarction and maternal vessel thrombosis.
Culture and serology [21]. — Routine cervical
cultures are not useful in the evaluation of RPL among
otherwise healthy women.
Autoantibodies and immune function [64-74]. — Many
studies have reported the presence of autoantibodies in
women with RPL .The pregnancy outcome of women with and
without antinuclear antibody (ANA) is the same hence routine
testing for ANA not recommended. Peripheral blood NK cells
are phenotypically and functionally different from uterine
NK (uNK) cells. There is no clear evidence that altered
peripheral blood NK cells are related to recurrent
miscarriage. Examining the relationship between uNK cell
numbers and future pregnancy outcome remains a research
field. Selection of appropriate tests for diagnosis of
immune-based RPL (HLA typing, mixed lymphocytotoxic antibody
tests, CD56+ cells and cytokine polymorphism) also requires
further investigation and validation.
Screening for diabetes — limited to women with
clinical manifestations of the disease.
Progesterone level [75] — Single or multiple serum
progesterone levels are not predictive of future pregnancy
outcome.
Endometrial biopsy [76] for luteal phase defect is
not predictive of fertility status and not recommended.
MANAGEMENT:
RPL is an inhomogeneous condition and hence specific
guidelines cannot be applicable in general to all. The
development of an optimal investigation and management
protocol depends on reaching a correct diagnosis of etiology
and directing specific treatment. Therapeutic
recommendations are largely based upon clinical experience
and data from observational studies. The overall live birth
rates after normal and abnormal diagnostic evaluations for
RPL are 77 and 71 percent, respectively [77].In all cases,
psychological support is vital[78,79].
• PARENTAL KARYOTYPE ABNORMALITY [80-85] — Couples
in whom chromosomal abnormalities are discovered in one or
either partners or the abortus are generally referred for
genetic counselling. They should receive information
regarding the probability of having a chromosomally normal
or abnormal conception in the future... The magnitude of
these risks varies according to the specific chromosomal
abnormality and the sex of the carrier parent.
Management options for these couples
• Prenatal genetic studies (amniocentesis or chorionic
villus sampling)
• In vitro fertilization (IVF) with preimplantation genetic
screening(PGT-A) Gamete donation (egg or sperm)
• Adoption
UTERINE ABNORMALITIES [86-91] — Uterine
abnormalities are managed surgically (hysteroscopically) if
correctable cause, such as a uterine septum, intrauterine
adhesions, or submucosal myoma.
There are no randomized trials evaluating pregnancy
outcome after surgical correction of uterine anomalies. In a
classic observational series, repair of septate uteri
reduced the abortion rate from 84 percent (before surgery)
to 12 percent (after surgery) using patients as their own
controls The value of prophylactic cervical cerclage in
women with a uterine anomaly, but no history of second
trimester pregnancy loss, is controversial. Cervical
cerclage is associated with potential hazards related to the
surgery and the risk of stimulating uterine contractions and
should be considered only in women who are likely to
benefit. Women with a history of second-trimester
miscarriage and suspected cervical weakness should be
monitored closely by serial cervical scans. In women with a
singleton pregnancy and a history of one second-trimester
miscarriage, an ultrasound-indicated cerclage should be
offered if a cervical length of 25 mm or less is detected by
transvaginal scan before 24 weeks of gestation. A
gestational carrier (surrogate) is an option for women with
irreparable uterine defects.
ANTIPHOSPHOLIPID SYNDROME [92]. — Aspirin and
heparin improve pregnancy outcome in women with APS with RPL.
LMWH appear to have additional qualities in preventing
adverse pregnancy outcome by their anti-inflammatory and
proangiogenic properties.
SUSPECTED IMMUNOLOGIC DYSFUNCTION [93-101]— No
alloimmune mechanism has been proven to cause RPL
.Immunologic treatments for unexplained RPL are not
effective, and may even be harmful as proven in systematic
reviews and should use only in the setting of a clinical
trial regulated by an Institutional Review Board.
• Paternal cell immunization
• Third party donor cell immunization
• Trophoblast membrane infusion
• Intravenous immunoglobulins (IV Ig)
• Glucocorticoids— Glucocorticoids have several
anti-inflammatory effects, including suppression of NK cell
activity, but do not appear to be effective for preventing
RPL. Steroids for treatment of RPL has been abandoned
because of uncertain efficacy and increase in complications,
such as preterm premature rupture of membranes, gestational
diabetes, and maternal hypertension
THYROID DYSFUNCTION AND DIABETES MELLITUS [102,103].
—
• Women with overt thyroid disease or diabetes mellitus
should be treated, as medically appropriate, since these
disorders can result in serious sequelae.
• Women with elevated serum thyroid peroxidase antibody
concentrations are at high risk of developing hypothyroidism
in the first trimester and autoimmune thyroiditis
postpartum, and should be followed appropriately
• Euthyroid women with high serum thyroid peroxidase
antibody concentrations may benefit from treatment with
levothyroxine (50 mcg daily) during pregnancy as it may
reduce the risk of miscarriage and preterm birth although
more trials are required.
POLYCYSTIC OVARY SYNDROME [104]. — The miscarriage
rate in women with polycystic ovary syndrome (PCOS) is 20 to
40 percent, higher than the baseline rate in the general
obstetric population Metformin has been used in women with
PCOS to decrease this risk, but the effectiveness of this
approach is unproven.
HYPERPROLACTINEMIA [105]. — Normal levels of
prolactin may play a significant role in maintaining early
pregnancy. A study of 64 hyperprolactinemic women with RPL
randomly assigned to bromocriptine therapy or no
bromocriptine found treatment was associated with a
significantly higher rate of successful pregnancy (86 versus
52 percent Prolactin levels during early pregnancy were
significantly greater in women who miscarried.
THROMBOPHILIA — Anticoagulation of women with
certain inherited thrombophilias may improve maternal
outcome (e.g., prevention of venous thromboembolism), but
controversial in RPL.
TREATMENT OPTIONS FOR UNEXPLAINED RPL — [106]. A
significant proportion of cases of RPL remain unexplained
despite detailed investigation. These women can be reassured
that the prognosis for a successful future pregnancy with
supportive care alone is almost 75%.
Several unproven treatments are often offered for
unexplained RPL
• Lifestyle modification — Eliminating use of tobacco
products, alcohol, and caffeine and reduction in body mass
index (for obese women) may improve chances of live birth
• Progesterone [106-110] — Therapeutic effect of
progesterone may be related to immune modulation it is
possible that earlier initiation of progesterone, such as
during the luteal phase, may improve outcome as shown by
small studies.
Older metaanalysis including smaller heterogeneous
studies confounded by fetal factors showed a beneficial
effect but a large trial comparing first-trimester vaginal
progesterone therapy or placebo showed no significant
difference
• Human menopausal gonadotropin [111] — an
observational study reported that controlled ovarian
stimulation with human menopausal gonadotropin (hMG)
appeared effective for endometrial defects in women with RPL
likely by correction of a luteal phase defect or a thicker
endometrium, leading to a better implantation.
• Human chorionic gonadotropin (118) — HCG is
critical to early pregnancy, ensuring active maintenance of
steroid production from the corpus luteum and for
endometrial preparation to facilitate implantation. Although
HCG has shown to improve LBR in systematic reviews but there
is insufficient evidence to recommend the use of hCG to
prevent pregnancy loss in women with a history of
unexplained RPL. However hCG also had detrimental effects on
decidualization in vitro. There are evidences both for and
against its use, so it should be offered to women only
within a research trial. Large randomized controlled trials
to identify subgroups which are likely to benefit are
needed.
• In vitro fertilization and preimplantation genetic
diagnosis (PGT-A) [112-116]. — Studies evaluating the
value of in vitro fertilization (IVF) in women with RPL have
yielded mixed results. Embryos of women with unexplained RPL
have a higher incidence of aneuploidy. In a retrospective
cohort study of 300 women with RPL, the pregnancy, live
birth, and miscarriage rates were similar for women who
underwent IVF with preimplantation screening (PGS) and women
who elected expectant management other drawbacks include
need for IVF, cost involved and issues related to PGT-A like
mosaicism.
• Oocyte donation [117] — Poor quality oocytes may
be responsible for 25 percent of pregnancy losses Ovum
donation can overcome this problem and has been associated
with a live birth rate of 88 percent in women with RPL.
• Surrogacy — a gestational carrier may be
considered in RPL not associated with recurrent embryonic
aneuploidy or obvious intrinsic gamete factors (e.g., single
gene defects, diminished oocyte and embryo quality).
FUTURE PREGNANCY PROGNOSIS
• Continued pregnancy loss [119,120] — the
greatest risk of recurrent loss occurs during the period up
to the time of previous miscarriage. The likelihood of
successful pregnancy in women with a history of recurrent
pregnancy loss (RPL) was 67-75 percent at 5 years.
Increasing maternal age and number of miscarriages are
associated with a poorer prognosis.
• Other obstetric issues. [121,122].— Women with a
history of RPL who become pregnant may be at higher risk for
developing fetal growth restriction and premature delivery,
but not for gestational hypertension or diabetes
Table IV: Comparison of Guidelines for the
Investigation and Treatment of Recurrent Pregnancy Loss
|
Investigation or Treatment |
ASRM Guidelines |
RCOG Guidelines |
ESHRE Guidelines(2017) |
|
Parental karyotyping |
Recommended |
Recommended only if POC shows unbalanced
translocation |
Recommended only after individual risk
assessment |
|
POC karyotyping |
Recommended |
Recommended after 2 miscarriages |
Trials required |
|
aCGH preferable |
|
APS assessment (ACA and LA) |
Recommended |
Recommended |
Recommended |
|
Treatment of APS with heparin and aspirin |
Recommended |
Recommended |
Recommended |
|
Thyroid function |
Recommended |
Recommended |
Recommended |
|
Treatment of Overt Hypothyroidism with
levothyroxine |
|
Recommended |
Recommended |
|
Treatment of Subclinical hypothyroidism |
|
Recommended |
Need more trials, Inconsistent evidence |
|
Glucose intolerance testing in PCO |
|
Insufficient evidence |
Not Recommended for RPL prognosis |
|
Metformin in RPL with PCO |
|
Insufficient evidence |
Insufficient evidence |
|
Prolactin estimation |
Recommended |
|
Not recommended in absence of clinical signs |
|
Bromocriptine for Hyperprolactinemia |
|
|
Recommended |
|
Ovarian reserve testing |
|
|
Insufficient evidence |
|
Uterine cavity assessment |
Insufficient evidence |
Recommended |
Recommended (3D US/Sono HG)) |
|
Resection of uterine septum |
Can be considered |
Insufficient evidence |
More trials needed |
|
Hysteroscopic polypectomy/Myomectomy |
Can be considered |
|
Not recommended |
|
Hysteroscopic adhesiolysis |
Can be considered |
|
Insufficient evidence |
|
Serial cervical USG surveillance in suspected
incompetence |
|
Recommended |
Recommended |
|
Cervical cerclage for second trimester loss |
|
Ultrasound indicated |
|
|
Luteal Phase Insufficiency testing |
Insufficient evidence |
Not recommended |
Not recommended |
|
Progesterone supplementation |
Insufficient evidence |
Insufficient evidence |
Insufficient evidence- More RCTs required |
|
hCG supplementation |
|
Insufficient evidence |
Insufficient evidence |
|
Bacterial vaginosis |
Not recommended |
Insufficient evidence |
|
|
Hereditary thrombophilias |
Not recommended |
Recommended for second trimester losses |
Recommended in research settings or if
additional risk factors |
|
Anticoagulants for hereditary thrombophilia |
|
Insufficient evidence |
Insufficient evidence |
|
TORCH Testing |
Not recommended |
Not recommended |
Not recommended |
|
Alloimmune testing |
Not recommended |
Not recommended |
Insufficient evidence |
|
(HLA, Peripheral blood NK cells, Cytokine
polymorphism) |
|
ANA |
|
|
For explanatory purpose |
|
Immunotherapy(LIT/IVIg) |
Not recommended |
Not recommended |
Insufficient evidence |
|
Tender loving care |
Recommended |
Insufficient evidence |
Recommended |
|
Obesity, smoking, alcohol |
|
|
Recommended |
|
Folic acid for hyperhomocysteinemia |
|
|
Insufficient evidence |
|
Preconceptional Vitamin D supplementation |
|
|
Recommended based on significant prevalence in
RPL |
|
Steroids |
Not recommended |
Not recommended |
Not recommended |
|
G CSF / Intralipids /
Heparin/Aspirin/Endometrial scratching for
Unexplained RPL |
|
|
Not recommended |
|
Male partner life style factors. |
|
|
Recommended |
|
Assessing sperm DNA fragmentation |
Insufficient evidence |
|
Considered for explanatory purposes, based on
indirect evidence |
|
Antioxidants for men |
|
|
Insufficient evidence |
ASRM: American Society of Reproductive Medicine; RCOG: Royal
College of Obstetricians; ESHRE: European Society of Human
Reproduction and Embryology
SUMMARY AND RECOMMENDATIONS
●Recurrent pregnancy loss (RPL) refers to the occurrence
of three or more consecutive losses of clinically recognized
pregnancies prior to the 20th week of gestation (excluding
ectopic, molar, and biochemical pregnancies). It may be
primary or secondary.
●0.4 to 1 percent of women have three consecutive pregnancy
losses.
● Chromosomal abnormalities are the most common cause of
sporadic early pregnancy loss (50 %). 3 to 5 % of couples
with RPL have a major chromosomal rearrangement (vs. 0.7
percent of the general population); usually a balanced
translocation.
●Uterine abnormalities, both acquired and congenital have
been reported to be responsible for 10 to 50 percent of RPL
in small studies.
●Pregnancy loss is one of the diagnostic criteria for
antiphospholipid syndrome.
●Endocrine factors may account for some cases of RPL.
●There is no strong evidence showing a relationship between
RPL and occupational factors, stress, or mild exposure to
most environmental chemicals.
●RPL typically occurs at a similar gestational age in
consecutive pregnancies. The recurrence risk increases as
gestational age at the time of loss increases.
●Evaluation of women for RPL may be recommended after two or
three consecutive miscarriages depending on other factors
like age.
●A detailed history and physical examination should guide
the clinician regarding probable etiology and tailor
diagnostic investigations and management in RPL.
●The following initial evaluation may
be recommended as per need:
•Sonohysterography/3D USG for assessment of uterine
abnormalities
•Anticardiolipin antibody (IgG and IgM) titre and lupus
anticoagulant performed twice, six to eight weeks apart
•Thyroid stimulating hormone (TSH) and thyroid peroxidase
antibodies
•Parental karyotype and karyotype of the abortus if the
above examinations are normal.
Additional testing depends upon the diagnosis suggested by
the history, physical examination, and laboratory results.
• Couples with chromosomal abnormalities in one or both
partners or the abortus are generally referred for genetic
counselling.
• Correctable uterine abnormalities such as a uterine septum
or intrauterine adhesions may be managed hysteroscopically.
• For women with unexplained RPL, there is not enough
evidence that use of vaginal progesterone or HCG improves
live birth rates.
• Immunotherapy or glucocorticoids are not effective for RPL
and may be harmful.
• Women with hyperprolactinemia and RPL should be treated.
• For unexplained RPL low risk, simple, and less expensive
interventions should be preferred over more complex and
expensive options.
• Women with a history of RPL who become pregnant may be at
higher risk for developing fetal growth restriction and
premature delivery.
REFERENCES
1. Christiansen OB, Nybo Andersen AM, Bosch E, et al.
Evidence-based investigations and treatments of recurrent
pregnancy loss. Fertil Steril 2005; 83:821.
2. Practice Committee of American Society for Reproductive
Medicine. Definitions of infertility and recurrent pregnancy
loss: a committee opinion. Fertil Steril 2013; 99:63.
3. Jauniaux E, Farquharson RG, Christiansen OB, Exalto N.
Evidence-based guidelines for the investigation and medical
treatment of recurrent miscarriage. Hum Reprod 2006;
21:2216.
4. Greentop Guideline 17. Recurrent Miscarriage,
investigation and treatment of couples. Royal College of
Obstetricians and Gynaecologists, 2011.
5. Kolte AM, van Oppenraaij RH, Quenby S, et al.
Non-visualized pregnancy losses are prognostically important
for unexplained recurrent miscarriage. Hum Reprod 2014;
29:931.
6. Kolte AM, Bernardi LA, Christiansen OB, et al.
Terminology for pregnancy loss prior to viability: a
consensus statement from the ESHRE early pregnancy special
interest group. Hum Reprod 2015; 30:495.
7. Ansari AH, Kirkpatrick B. Recurrent pregnancy loss. An
update. J Reprod Med 1998; 43:806.
8. Paukku M, Tulppala M, Puolakkainen M, et al. Lack of
association between serum antibodies to Chlamydia
trachomatis and a history of recurrent pregnancy loss.
Fertil Steril 1999; 72:427.
9. Salat-Baroux J. [Recurrent spontaneous abortions]. Reprod
Nutr Dev 1988; 28:1555.
10. Edmonds DK, Lindsay KS, Miller JF, et al. Early
embryonic mortality in women. Fertil Steril 1982; 38:447.
11. Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of
early loss of pregnancy. N Engl J Med 1988; 319:189.
12. Nybo Andersen AM, Wohlfahrt J, Christens P, et al.
Maternal age and fetal loss: population based register
linkage study. BMJ 2000; 320:1708.
13. Stirrat GM. Recurrent miscarriage. Lancet 1990; 336:673.
14. Franssen MT, Korevaar JC, Leschot NJ, et al. Selective
chromosome analysis in couples with two or more
miscarriages: case-control study. BMJ 2005; 331:137.
15. De Braekeleer M, Dao TN. Cytogenetic studies in couples
experiencing repeated pregnancy losses. Hum Reprod 1990;
5:519.
16. Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai
G. Karyotype of the abortus in recurrent miscarriage. Fertil
Steril 2001;75:678–82
17. Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic
karyotype of abortuses in relation to the number of previous
miscarriages. Fertil Steril 2000; 73:300–4.)
18. Lockwood CJ, Romero R, Feinberg RF, Clyne LP, Coster B,
Hobbins JC. The prevalence and biologic significance of
lupus anticoagulant and anticardiolipin antibodies in a
general obstetric population. Am J Obstet Gynecol 1989;
161:369–73?
19. Pattison NS, Chamley LW, McKay EJ, Liggins GC, Butler
WS. Antiphospholipid antibodies in pregnancy: prevalence and
clinical association.Br J Obstet Gynaecol 1993; 100:909–13.
20. Hill JA, Choi BC. Maternal immunological aspects of
pregnancy success and failure. J Reprod Fertil Suppl 2000;
55:91.
21. American College of Obstetricians and Gynecologists.
ACOG practice bulletin. Management of recurrent pregnancy
loss. Number 24, February 2001.
22. Dosiou C, Giudice LC. Natural killer cells in pregnancy
and recurrent pregnancy loss: endocrine and immunologic
perspectives. Endocr Rev 2005; 26:44.
23. Laird SM, Tuckerman EM, Cork BA, et al. A review of
immune cells and molecules in women with recurrent
miscarriage. Hum Reprod Update 2003; 9:163.
24. Sotiriadis A, Makrigiannakis A, Stefos T, et al.
Fibrinolytic defects and recurrent miscarriage: a systematic
review and meta-analysis. Obstet Gynecol 2007; 109:1146.
25. Laude I, Rongières-Bertrand C, Boyer-Neumann C, et al.
Circulating procoagulant microparticles in women with
unexplained pregnancy loss: a new insight. Thromb Haemost
2001; 85:18.
26. Acién P, Acién M, Sánchez-Ferrer M. Complex
malformations of the female genital tract. New types and
revision of classification. Hum Reprod 2004; 19:2377.
27. Devi Wold AS, Pham N, Arici A. Anatomic factors in
recurrent pregnancy loss. Semin Reprod Med 2006; 24:25.
28. Homer HA, Li TC, Cooke ID. The septate uterus: a review
of management and reproductive outcome. Fertil Steril 2000;
73:1.
29. Proctor JA, Haney AF. Recurrent first trimester
pregnancy loss is associated with uterine septum but not
with bicornuate uterus. Fertil Steril 2003; 80:1212.
30. Paulson RJ. Hormonal induction of endometrial
receptivity. Fertil Steril 2011; 96:530.
31. Lessey BA. Assessment of endometrial receptivity. Fertil
Steril 2011; 96:522.
32. Li TC, Tuckerman EM, Laird SM. Endometrial factors in
recurrent miscarriage. Hum Reprod Update 2002; 8:43.
33. Lucas ES, Dyer NP, Murakami K, et al. Loss of
Endometrial Plasticity in Recurrent Pregnancy Loss. Stem
Cells 2016; 34:346.
34. Savitz DA, Sonnenfeld NL, Olshan AF. Review of
epidemiologic studies of paternal occupational exposure and
spontaneous abortion. Am J Ind Med 1994; 25:361.
35. Bellver J, Rossal LP, Bosch E, et al. Obesity and the
risk of spontaneous abortion after oocyte donation. Fertil
Steril 2003; 79:1136.
36. Gopalkrishnan K, Padwal V, Meherji PK, et al. Poor
quality of sperm as it affects repeated early pregnancy
loss. Arch Androl 2000; 45:111.
37. Carrell DT, Wilcox AL, Lowy L, et al. Elevated sperm
chromosome aneuploidy and apoptosis in patients with
unexplained recurrent pregnancy loss. Obstet Gynecol 2003;
101:1229.
38. Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al.
Relationship between sperm aneuploidy, sperm DNA integrity,
chromatin packaging, traditional semen parameters, and
recurrent pregnancy loss. Fertil Steril 2016; 105:58.
39. . Leitich H,Kiss H.Asymptomatic bacterial vaginosis and
intermediate flora as risk factors for adverse pregnancy
outcome. Best Pract Res Clin Obstet Gynaecol 2007;
21:375–90.
40. Llahi-Camp JM, Rai R, Ison C, Regan L,Taylor-Robinson D.
Association of bacterial vaginosis with a history of second
trimester miscarriage. Hum Reprod 1996; 11:1575–8.
41. Ralph SG, Rutherford AJ, Wilson JD. Influence of
bacterial vaginosis on conception and miscarriage in the
first trimester: cohort study. BMJ 1999; 319:220–3.
42. Trout SW, Seifer DB. Do women with unexplained recurrent
pregnancy loss have higher day 3 serum FSH and estradiol
values? Fertil Steril 2000; 74:335.
43. Bombell S, McGuire W. Cytokine polymorphisms in women
with recurrent pregnancy loss: meta-analysis. Aust N Z J
Obstet Gynaecol 2008; 48:147.
44. Schweikert A, Rau T, Berkholz A, et al. Association of
progesterone receptor polymorphism with recurrent abortions.
Eur J Obstet Gynecol Reprod Biol 2004; 113:67.
45. Bahia W, Finan RR, Al-Mutawa M, et al. Genetic variation
in the progesterone receptor gene and susceptibility to
recurrent pregnancy loss: a case-control study. BJOG 2018;
125:729.
46. Clark DA, Daya S, Coulam CB, Gunby J. Implication of
abnormal human trophoblast karyotype for the evidence-based
approach to the understanding, investigation, and treatment
of recurrent spontaneous abortion. The Recurrent Miscarriage
Immunotherapy Trialists Group. Am J Reprod Immunol 1996;
35:495?
47. Hassold T, Jacobs PA, Pettay D. Cytogenetic studies of
couples with repeated spontaneous abortions of known
karyotype. Genet Epidemiol 1988; 5:65.
48. Schaeffer AJ, Chung J, Heretis K, et al. Comparative
genomic hybridization-array analysis enhances the detection
of aneuploidies and submicroscopic imbalances in spontaneous
miscarriages. Am J Hum Genet 2004; 74:1168?
49. Fritz B, Hallermann C, Olert J, et al. Cytogenetic
analyses of culture failures by comparative genomic
hybridisation (CGH)-Re-evaluation of chromosome aberration
rates in early spontaneous abortions. Eur J Hum Genet 2001;
9:539.
50. ACOG Committee Opinion No. 446: array comparative
genomic hybridization in prenatal diagnosis. Obstet Gynecol
2009; 114:1161.
51. Goldberg JM, Falcone T, Attaran M. Sonohysterographic
evaluation of uterine abnormalities noted on
hysterosalpingography. Hum Reprod 1997; 12:2151.
52. Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic
accuracy of sonohysterography, transvaginal sonography, and
hysterosalpingography in patients with uterine cavity
diseases. Fertil Steril 2000; 73:406.
53. Keltz MD, Olive DL, Kim AH, Arici A. Sonohysterography
for screening in recurrent pregnancy loss. Fertil Steril
1997; 67:670.
54. Reuter KL, Daly DC, Cohen SM. Septate versus bicornuate
uteri: errors in imaging diagnosis. Radiology 1989; 172:749.
55. Malhotra N, Sood M. Role of hysteroscopy in infertile
women. J Indian Med Assoc 1997; 95:499, 525.
56. Pellerito JS, McCarthy SM, Doyle MB, et al. Diagnosis of
uterine anomalies: relative accuracy of MR imaging,
endovaginal sonography, and hysterosalpingography. Radiology
1992; 183:795.
57. Bermejo C, Martínez Ten P, Cantarero R, et al.
Three-dimensional ultrasound in the diagnosis of Müllerian
duct anomalies and concordance with magnetic resonance
imaging. Ultrasound Obstet Gynecol 2010; 35:593.
58. Roubey RA. Update on antiphospholipid antibodies. Curr
Opin Rheumatol 2000; 12:374.
59. Vinatier D, Dufour P, Cosson M, Houpeau JL.
Antiphospholipid syndrome and recurrent miscarriages. Eur J
Obstet Gynecol Reprod Biol 2001; 96:37.
60. Coulam CB, Branch DW, Clark DA et al. American Society
for Reproductive Immunology report of the Committee for
establishing criteria for diagnosis of reproductive
autoimmune syndrome. Am J Reprod Immunol 1999; 41:121–32?
61. Negro R, Schwartz A, Gismondi R, et al. Increased
pregnancy loss rate in thyroid antibody negative women with
TSH levels between 2.5 and 5.0 in the first trimester of
pregnancy. J Clin Endocrinol Metab 2010; 95:E44.
62. Chen L, Hu R. Thyroid autoimmunity and miscarriage: a
meta-analysis. Clin Endocrinol (Oxf) 2011; 74:513.
63. Thangaratinam S, Tan A, Knox E, et al. Association
between thyroid autoantibodies and miscarriage and preterm
birth: meta-analysis of evidence. BMJ 2011; 342:d2616.
64. Cervera R, Balasch J. Autoimmunity and recurrent
pregnancy losses. Clin Rev Allergy Immunol 2010; 39:148.
65. Harger JH, Rabin BS, Marchese SG. The prognostic value
of antinuclear antibodies in women with recurrent pregnancy
losses: a prospective controlled study. Obstet Gynecol 1989;
73:419.
66. Coulam CB. Immunologic tests in the evaluation of
reproductive disorders: a critical review. Am J Obstet
Gynecol 1992; 167:1844?
67. Laird SM, Tuckerman EM, Cork BA, et al. A review of
immune cells and molecules in women with recurrent
miscarriage. Hum Reprod Update 2003; 9:163.
68. Clark DA. Is there any evidence for immunologically
mediated or immunologically modifiable early pregnancy
failure? J Assist Reprod Genet 2003; 20:63.
69. Moffett A, Regan L, Braude P. Natural killer cells,
miscarriage, and infertility. BMJ 2004; 329:1283–5.
70. Wold AS, Arici A. Natural killer cells and reproductive
failure. Curr Opin Obstet Gynecol 2005; 17:237–41.
71. Rai R, Sacks G, Trew G. Natural killer cells and
reproductive failure – theory, practice and prejudice. Hum
Reprod 2005; 20:1123–6.
72. Le Bouteiller P, Piccinni MP. Human NK cells in pregnant
uterus: why there? Am J Reprod Immunol 2008; 59:401–6?
73. Tuckerman E, Laird SM, Prakash A, LiTC. Prognostic value
of the measurement of uterine natural killer cells in the
endometrium of women with recurrent miscarriage. Hum Reprod
2007; 22:2208–13.
74. Bombell S, McGuire W. Cytokine polymorphisms in women
with recurrent pregnancy loss: meta-analysis. Aust N Z J
Obstet Gynaecol 2008; 48:147–54.
75. Ogasawara M, Kajiura S, Katano K, et al. Are serum
progesterone levels predictive of recurrent miscarriage in
future pregnancies? Fertil Steril 1997; 68:806.
76. Peters AJ, Lloyd RP, Coulam CB. Prevalence of
out-of-phase endometrial biopsy specimens. Am J Obstet
Gynecol 1992; 166:1738?
77. Harger JH, Archer DF, Marchese SG, et al. Etiology of
recurrent pregnancy losses and outcome of subsequent
pregnancies. Obstet Gynecol 1983; 62:574.
78. Liddell HS, Pattison NS, Zanderigo A. Recurrent
miscarriage--outcome after supportive care in early
pregnancy. Aust N Z J Obstet Gynaecol 1991; 31:320.
79. Clifford K, Rai R, Regan L. Future pregnancy outcome in
unexplained recurrent first trimester miscarriage. Hum
Reprod 1997; 12:387.
80. Laurino MY, Bennett RL, Saraiya DS, et al. Genetic
evaluation and counselling of couples with recurrent
miscarriage: recommendations of the National Society of
Genetic Counsellors. J Genet Couns 2005; 14:165.
81. Egozcue J, Santaló J, Giménez C, et al. Preimplantation
genetic diagnosis. Mol Cell Endocrinol 2000; 166:21.
82. Munné S, Cohen J, Sable D. Preimplantation genetic
diagnosis for advanced maternal age and other indications.
Fertil Steril 2002; 78:234.
83. Werlin L, Rodi I, DeCherney A, et al. Preimplantation
genetic diagnosis as both a therapeutic and diagnostic tool
in assisted reproductive technology. Fertil Steril 2003;
80:467.
84. Otani T, Roche M, Mizuike M, et al. Preimplantation
genetic diagnosis significantly improves the pregnancy
outcome of translocation carriers with a history of
recurrent miscarriage and unsuccessful pregnancies. Reprod
Biomed Online 2006; 13:869.
85. Mastenbroek S, Twisk M, van Echten-Arends J, et al. In
vitro fertilization with preimplantation genetic screening.
N Engl J Med 2007; 357:9.
86. March CM, Israel R. Gestational outcome following
hysteroscopic lysis of adhesions. Fertil Steril 1981;
36:455.
87. Kutteh WH. Antiphospholipid antibody-associated
recurrent pregnancy loss: treatment with heparin and
low-dose aspirin is superior to low-dose aspirin alone. Am J
Obstet Gynecol 1996; 174:1584?
88. March CM, Israel R. Hysteroscopic management of
recurrent abortion caused by septate uterus. Am J Obstet
Gynecol 1987; 156:834?
89. Patton PE, Novy MJ, Lee DM, Hickok LR. The diagnosis and
reproductive outcome after surgical treatment of the
complete septate uterus, duplicated cervix and vaginal
septum. Am J Obstet Gynecol 2004; 190:1669?
90. Mollo A, De Franciscis P, Colacurci N, et al.
Hysteroscopic resection of the septum improves the pregnancy
rate of women with unexplained infertility: a prospective
controlled trial. Fertil Steril 2009; 91:2628.
91. Tomaževič T, Ban-Frangež H, Virant-Klun I, et al.
Septate, subseptate and arcuate uterus decrease pregnancy
and live birth rates in IVF/ICSI. Reprod Biomed Online 2010;
21:700.
92. Recurrent Pregnancy Loss, Guideline of the European
Society of Human Reproduction and Embryology, NOVEMBER 2017
ESHRE Early Pregnancy Guideline Development Group
93. Wong LF, Porter TF, Scott JR. Immunotherapy for
recurrent miscarriage. Cochrane Database Syst Rev 2014;
CD000112.
94. Hutton B, Sharma R, Fergusson D, et al. Use of
intravenous immunoglobulin for treatment of recurrent
miscarriage: a systematic review. BJOG 2007; 114:134.
95. Laskin CA, Bombardier C, Hannah ME, et al. Prednisone
and aspirin in women with autoantibodies and unexplained
recurrent fetal loss. N Engl J Med 1997; 337:148.
96. Regan L, Rai R. Epidemiology and the medical causes of
miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol
2000; 14:839.
97. Ogasawara M, Aoki K. Successful uterine steroid therapy
in a case with a history of ten miscarriages. Am J Reprod
Immunol 2000; 44:253?
98. Christiansen OB, Larsen EC, Egerup P, et al. Intravenous
immunoglobulin treatment for secondary recurrent
miscarriage: a randomised, double-blind, placebo-controlled
trial. BJOG 2015; 122:500.
99. Clark DA, Coulam CB, Stricker RB. Is intravenous
immunoglobulins (IVIG) efficacious in early pregnancy
failure? A critical review and meta-analysis for patients
who fail in vitro fertilization and embryo transfer (IVF). J
Assist Reprod Genet 2006; 23:1.
100. Ata B, Tan SL, Shehata F, et al. A systematic review of
intravenous immunoglobulin for treatment of unexplained
recurrent miscarriage. Fertil Steril 2011; 95:1080.
101. Egerup P, Lindschou J, Gluud C, et al. The Effects of
Intravenous Immunoglobulins in Women with Recurrent
Miscarriages: A Systematic Review of Randomised Trials with
Meta-Analyses and Trial Sequential Analyses Including
Individual Patient Data. PLoS One 2015; 10:e0141588.
102. Marqusee E, Hill JA, Mandel SJ. Thyroiditis after
pregnancy loss. J Clin Endocrinol Metab 1997; 82:2455.
103. Negro R, Formoso G, Mangieri T, et al. Levothyroxine
treatment in euthyroid pregnant women with autoimmune
thyroid disease: effects on obstetrical complications. J
Clin Endocrinol Metab 2006; 91:2587.
104. Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L.
Pregnancy outcomes among women with polycystic ovary
syndrome treated with metformin. Hum Reprod 2002; 17:2858.
105. Hirahara F, Andoh N, Sawai K, et al. Hyperprolactinemic
recurrent miscarriage and results of randomized
bromocriptine treatment trials. Fertil Steril 1998; 70:246.
106. Coomarasamy A, Williams H, Truchanowicz E, et al. A
Randomized Trial of Progesterone in Women with Recurrent
Miscarriages. N Engl J Med 2015; 373:2141.
107. Haas DM, Ramsey PS. Progestogen for preventing
miscarriage. Cochrane Database Syst Rev 2013; CD003511.
108. Coomarasamy A, Truchanowicz EG, Rai R. Does first
trimester progesterone prophylaxis increase the live birth
rate in women with unexplained recurrent miscarriages? BMJ
2011; 342:d1914.
109. Saccone G, Schoen C, Franasiak JM, et al.
Supplementation with progestogens in the first trimester of
pregnancy to prevent miscarriage in women with unexplained
recurrent miscarriage: a systematic review and meta-analysis
of randomized, controlled trials. Fertil Steril 2017;
107:430.
110. Choi BC, Polgar K, Xiao L, Hill JA. Progesterone
inhibits in-vitro embryotoxic Th1 cytokine production to
trophoblast in women with recurrent pregnancy loss. Hum
Reprod 2000; 15 Suppl 1:46.
111. Li TC, Ding SH, Anstie B, et al. Use of human
menopausal gonadotropins in the treatment of endometrial
defects associated with recurrent miscarriage: preliminary
report. Fertil Steril 2001; 75:434.
112. Balasch J, Creus M, Fabregues F, et al. In-vitro
fertilization treatment for unexplained recurrent abortion:
a pilot study. Hum Reprod 1996; 11:1579.
113. Pellicer A, Rubio C, Vidal F, et al. In vitro
fertilization plus preimplantation genetic diagnosis in
patients with recurrent miscarriage: an analysis of
chromosome abnormalities in human preimplantation embryos.
Fertil Steril 1999; 71:1033.
114. Simón C, Rubio C, Vidal F, et al. Increased chromosome
abnormalities in human preimplantation embryos after
in-vitro fertilization in patients with recurrent
miscarriage. Reprod Fertil Dev 1998; 10:87.
115. Raziel A, Herman A, Strassburger D, et al. The outcome
of in vitro fertilization in unexplained habitual aborters
concurrent with secondary infertility. Fertil Steril 1997;
67:88.
116. Murugappan G, Shahine LK, Perfetto CO, et al. Intent to
treat analysis of in vitro fertilization and preimplantation
genetic screening versus expectant management in patients
with recurrent pregnancy loss. Hum Reprod 2016; 31:1668.
117. Remohí J, Gallardo E, Levy M, et al. Oocyte donation in
women with recurrent pregnancy loss. Hum Reprod 1996;
11:2048.
118. Morley LC, Simpson N, Tang T. Human chorionic
gonadotrophin (hCG) for preventing miscarriage. Cochrane
Database Syst Rev 2013; 31; 1:CD008611.)
119. Lund M, Kamper-Jørgensen M, Nielsen HS, et al.
Prognosis for live birth in women with recurrent
miscarriage: what is the best measure of success? Obstet
Gynecol 2012; 119:37.
120. Brigham SA, Conlon C, Farquharson RG. A longitudinal
study of pregnancy outcome following idiopathic recurrent
miscarriage. Hum Reprod 1999; 14:2868.
121. Field K, Murphy DJ. Perinatal outcomes in a subsequent
pregnancy among women who have experienced recurrent
miscarriage: a retrospective cohort study. Hum Reprod 2015;
30:1239.
122. Jivraj S, Anstie B, Cheong YC, et al. Obstetric and
neonatal outcome in women with a history of recurrent
miscarriage: a cohort study. Hum Reprod 2001; 16:102.
|
|
