| |
|
|
Author: Enrique Hernandez, MD, FACOG, FACS,
Chairman, Department of Obstetrics and Gynecology, Director
of Gynecologic Oncology, Abraham Roth Professor of
Obstetrics, Gynecology and Reproductive Science, Professor
of Pathology, Temple University Hospital, Temple University
School of Medicine
Background
Gestational trophoblastic disease (GTD) can
be benign or malignant. Histologically, it is classified
into hydatidiform mole, invasive mole (chorioadenoma
destruens), choriocarcinoma, and placental site
trophoblastic tumor (PSTT). Those that invade locally or
metastasize are collectively known as gestational
trophoblastic neoplasia (GTN). Hydatidiform mole is the most
common form of gestational trophoblastic neoplasia (see
Image 1). While invasive mole and choriocarcinoma are
malignant, a hydatidiform mole can behave in a malignant or
benign fashion.
No methods exist to accurately predict the
clinical behavior of a hydatidiform mole by histopathology.
The clinical course is defined by the patient's serum human
chorionic gonadotropin (HCG) curve after evacuation of the
mole. In 80% of patients with a benign hydatidiform mole,
serum HCG titers steadily drop to normal within 8-12 weeks
after evacuation of the molar pregnancy. In the other 20% of
patients with a malignant hydatidiform mole, serum HCG
titers either rise or plateau.
The official International Federation of
Gynecology and Obstetrics staging of gestational trophoblastic neoplasia is as follows:
-
Stage I – Confined to the uterus
-
Stage II – Limited to the genital
structures
-
Stage III – Lung metastases
-
Stage IV – Other metastases
Each stage is sub-classified further
according to a prognostic scoring index. If the risk factors
are unknown, no substage is assigned. If the prognostic
score is 7 or less, the substage is A (e.g., IIIA is equal
to lung metastasis with a prognostic score of 7 or less). If
the prognostic score is 8 or greater, the substage is B.
The currently used prognostic scoring index
is a modification of the World Health Organization (WHO)
classification. It provides points for the presence of a
number of prognostic factors, as follows:
-
Age 40 years or older = 1 point
-
Antecedent pregnancy terminated in
abortion = 1 point
-
Antecedent full-term pregnancy = 2 points
-
Interval of 4 months to less than 7
months between antecedent pregnancy and start of
chemotherapy = 1 point
-
Interval of 7-12 months between
antecedent pregnancy and start of chemotherapy = 2
points
-
Interval of more than 12 months between
antecedent pregnancy and start of chemotherapy = 4
points
-
Beta-HCG level in serum is 1000 mIU/mL
but less than 10,000 mIU/mL = 1 point
-
Beta-HCG level in serum is 10,000 mIU/mL
but less than 100,000 mIU/mL = 2 points
-
Beta-HCG level in serum is 100,000 mIU/mL
or greater = 4 points
-
Largest tumor is 3 cm but less than 5 cm
= 1 point
-
Largest tumor is 5 cm or greater = 2
points
-
Site of metastases is spleen or kidney =
1 point
-
Site of metastases is gastrointestinal
tract = 2 points
-
Site of metastases is brain or liver = 4
points
-
Number of metastases is 1-4 = 1 point
-
Number of metastases is 5-8 = 2 points
-
Number of metastases is more than 8 = 4
points
-
Prior chemotherapy with single drug = 2
points
-
Prior chemotherapy with multiple drugs =
4 points
Pathophysiology
Histologically, hydatidiform moles look like
placental tissue, but edema of the villi demonstrates
varying sizes. Proliferation of the trophoblast occurs, and
fetal blood vessels are lacking or are scarce.
If a fetus or fetal parts are present, this
is known as a partial or incomplete mole. Partial moles also
have malignant potential, but only 2% become malignant. An
invasive mole has the same histopathologic characteristics
of a hydatidiform mole, but invasion of the myometrium with
necrosis and hemorrhage occurs or pulmonary metastases are
present. Histologically, choriocarcinomas have no villi, but
they have sheets of trophoblasts and hemorrhage.
Choriocarcinomas are aneuploid and can be
heterozygous, depending on the type of pregnancy from which
the choriocarcinoma arose. If a hydatidiform mole preceded
the choriocarcinoma, the chromosomes are of paternal origin.
Maternal and paternal chromosomes are present if a term
pregnancy precedes the choriocarcinoma. Of choriocarcinomas,
50% are preceded by a hydatidiform mole, 25% by an abortion,
and the other 25% by a full-term pregnancy.
Placental site trophoblastic tumor is a rare
form of gestational trophoblastic neoplasm, with slightly
more than 200 cases reported in the literature. In patients
with PSTT, intermediate trophoblasts are found infiltrating
the myometrium without causing tissue destruction. The
intermediate trophoblasts contain human placental lactogen
(HPL). These patients have persistent low levels of serum
HCG (100-1000 mIU/mL). However, serum HCG titers as high as
58,000 mIU/ml have been reported in patients with placental
site trophoblastic tumors. The treatment of placental site
trophoblastic tumors is hysterectomy with ovarian
conservation. If the tumor recurs or metastases are present
at initial diagnosis, chemotherapy is administered with
variable results. Radiation therapy may provide local
control.
The most frequent sites of metastases of
malignant gestational trophoblastic neoplasm are the lungs,
lower genital tract, brain, liver, kidney, and
gastrointestinal tract.
Frequency
United States
Hydatidiform moles occur in 1 in 2000
deliveries, or 1 in 850 to 1 in 1300 pregnancies.
International
In Mexico, an incidence of 1 in 200
deliveries is reported, while an incidence of 1 in 120
deliveries is reported in Taiwan. Some believe these
international differences are due to differences in diet.
However, in some countries, these differences are due to
poor recording of the total number of deliveries, especially
if deliveries are normal and do not occur in a hospital.
Mortality/Morbidity
Patients who have a malignant hydatidiform
mole, an invasive mole, or a choriocarcinoma should undergo
a systematic search for metastases. Patients who have
metastases are classified as high-risk or low-risk according
to the National Institutes of Health classification. The
criteria for high-risk metastatic gestational trophoblastic
neoplasia include hepatic or brain metastasis, serum HCG
titers greater than 40,000 mIU/mL prior to the initiation of
chemotherapy, duration of disease longer than 4 months,
prior unsuccessful chemotherapy, and malignant gestational
trophoblastic neoplasia following a term pregnancy.
-
Patients with malignant non-metastatic or
metastatic low-risk gestational trophoblastic neoplasia
have an almost 100% probability of cure with
chemotherapy. The probability of cure after chemotherapy
for patients with metastatic high-risk gestational
trophoblastic neoplasia is approximately 75%.
-
The probability of a late recurrence
after the patient has been in remission (normal serum
beta-HCG titers) for 1 year is less than 1%.
Race
-
International reports are conflicting as
to whether ethnicity is an independent risk factor for
the development of gestational trophoblastic neoplasia.
-
In the United States, race does not
appear to be a risk factor.
Sex
Age
CLINICAL
History
-
Patients with a hydatidiform mole present
with signs and symptoms of pregnancy.
-
The most frequent symptom of
gestational trophoblastic neoplasia (GTN) is
abnormal uterine bleeding.
-
Patients have a history of
amenorrhea. Occasionally, the typical hydatid
vesicles (edematous villi) are passed through the
vagina.
-
Signs and symptoms of preeclampsia occur
in up to one third of patients.
-
Prolonged hyperemesis gravidarum is also
common.
-
Hyperthyroidism is found in up to 3% of
patients. This is due to the production of human molar
thyrotropin by the molar tissue and the similarities
between HCG and thyroid-stimulating hormone (TSH).
-
If metastases exist, signs and symptoms
associated with the metastatic disease, such as
hematuria, hemoptysis, abdominal pain, and neurologic
symptoms, may be present.
-
The more frequent use of early
obstetrical ultrasound has resulted in the earlier
diagnosis of hydatidiform mole prior to the onset of the
above signs and symptoms.
Physical
Suspect gestational trophoblastic
neoplasia when a positive pregnancy test result occurs
in the absence of a fetus.
Uterine size could be larger, smaller, or
equal to the estimated gestational age.
The identification of hydatid vesicles in
the vagina is diagnostic for hydatidiform mole.
Enlarged ovaries secondary to theca
lutein cysts are found in up to 20% of patients with
hydatidiform mole.
-
These cysts are the result of
stimulation of the ovaries by the high circulating
levels of HCG.
-
The cysts regress after evacuation of
the hydatidiform mole, but this process can take as
long as 12 weeks.
Causes
A hydatidiform mole occurs when a haploid
sperm fertilizes an egg that has no maternal chromosomes
and then duplicates its chromosomal complement.
-
Most complete hydatidiform moles are
46, XX, and all the chromosomes come from the male.
-
Of hydatidiform moles, 10-15% are 46,
XY. This occurs when 2 sperm, 1 carrying an X and
the other carrying a Y, fertilize an "empty" egg.
Partial moles are 69, XXY, and 2 sets of
chromosomes are of paternal origin.
Lab Studies
-
Serum HCG is elevated and frequently
higher than expected for the estimated gestational age.
A serum HCG greater than 100,000 mIU/mL should raise the
concern of gestational trophoblastic disease (GTD).
-
A CBC count may help detect anemia
secondary to vaginal bleeding.
-
Liver enzymes may become elevated in the
presence of metastasis to the liver.
Imaging Studies
·
-
CT and MRI are recommended if the
patient has malignant gestational trophoblastic
neoplasia (hydatidiform mole with metastasis to the
lungs, choriocarcinoma, or persistent hydatidiform
mole).
-
The lungs, lower genital tract,
brain, liver, kidney, and gastrointestinal tract are
frequent sites of metastases.
Procedures
-
Evacuation of the uterus is performed
with suction and sharp curettage.
-
The tissue is sent for
histopathologic examination.
-
Examination reveals a hydatidiform
mole (complete or partial) or a choriocarcinoma.
-
Rarely is a histopathologic diagnosis of
an invasive mole made on a dilation and curettage (D&C)
specimen because this requires the identification of
destructive invasion of the myometrium by the
trophoblasts. Scant or no myometrium is recovered on a
D&C specimen.
Histological Findings
Complete hydatidiform moles have edematous
placental villi, hyperplasia of the trophoblasts, and lack
or scarcity of fetal blood vessels.
In the incomplete or partial hydatidiform
mole, scalloping of the villi and trophoblastic inclusions
occur within the villi. Fetal blood vessels are present.
In a hydropic degeneration of a normal
pregnancy, edema of the villi is present, but no
trophoblastic hyperplasia. Ghost villi may be observed.
The invasive mole has the same appearance as
the hydatidiform mole, but the myometrium is invaded with
the presence of hemorrhage and tissue necrosis.
Although the choriocarcinoma has no chorionic
villi, it has sheets of trophoblasts, hemorrhage, and
necrosis. In the placental site trophoblastic tumors,
intermediate trophoblasts are found between myometrial
fibers, without tissue necrosis.
TREATMENT
Medical Care
-
Emergency department care involves
starting intravenous (IV) fluids (crystalloids) and
sending blood for type and antibody screen. Rh-negative
patients should receive anti–RhD immune globulin, if not
already immunized.
-
Patients with benign gestational
trophoblastic disease (GTD) do not require medical
therapy. Because 20% of patients with hydatidiform mole
develop malignant disease, such as persistent
hydatidiform mole with or without metastasis, some have
suggested the use of a prophylactic dose of methotrexate
(MTX) in noncompliant patients. However, observing
patients with weekly serum HCG titers is preferable, and
only patients with rising or plateauing titers, as
occurs in patients with malignant gestational
trophoblastic neoplasia (GTN), should be treated with
chemotherapy.
-
Patients with malignant nonmetastatic
gestational trophoblastic neoplasia or metastatic
low-risk gestational trophoblastic neoplasia are treated
with single-agent chemotherapy. Many in the United
States prefer MTX. However, actinomycin D can be used in
patients with poor liver function. During treatment, the
serum HCG titers are monitored every week. One
additional course of chemotherapy is administered after
a normal serum HCG titer. After 3-4 normal serum HCG
titers, the titers are followed once per month for 1
year. A switch from MTX to actinomycin D is made if the
patient receiving MTX for nonmetastatic or metastatic
low-risk gestational trophoblastic neoplasia develops
rising or plateauing serum HCG titers.
-
Patients with high-risk metastatic
gestational trophoblastic neoplasia are subdivided into
2 groups: those with a WHO score of less than 8 and
those with a score of 8 or higher and a high risk of
therapy failure.
·
-
In patients with a WHO score of less
than 8, a combination of MTX, actinomycin D, and
cyclophosphamide can be used. This is known as the
MAC regimen. This chemotherapeutic regimen is
administered every 19-21 days (from day 1 of the
previous chemotherapy cycle) until the serum HCG
titers normalize. In patients with a low WHO score,
one additional course of MAC is administered after a
normal serum HCG titer. Some prefer to treat these
patients with single-agent chemotherapy (MTX or
actinomycin) because their chances of achieving a
cure are high.
-
Patients with WHO scores of 8 or
higher are treated with a combination of etoposide,
MTX, and actinomycin D administered in the first
week of a 2-week cycle and cyclophosphamide and
vincristine administered in the second week. This is
known as the EMA-CO regimen. Some substitute
cisplatin and etoposide for cyclophosphamide and
vincristine during the second week. This is known as
the EMA-CE regimen. Some reserve the EMA-CE regimen
for patients in whom EMA-CO fails. Two additional
courses of EMA-CO or EMA-CE are administered after a
normal serum HCG titer in very high-risk patients.
Patients with metastasis to the brain receive whole
brain irradiation (3000 cGy) in combination with
chemotherapy. Corticosteroids (Decadron) with
systemic effect are administered to reduce brain
edema. Patients with liver metastasis are considered
for liver irradiation (2000 cGy).
Surgical Care
·
-
To avoid excessive bleeding, oxytocin
is administered intravenously at the initiation of
the suctioning of the uterine contents.
-
The largest possible suction curette
is used, usually a 10F or 12F.
FOLLOW-UP
Further Outpatient Care
-
In patients with benign gestational
trophoblastic disease (GTD), who do not require
chemotherapy, obtain follow-up serum HCG titers once per
week until 3-4 normal values are obtained. Then, obtain
them once per month for 6 months. Have patients use
reliable contraception, such as oral contraceptives or
depot progesterone injections, during the period of
follow-up care.
-
Patients with malignant gestational
trophoblastic neoplasia should have follow-up serum HCG
titers once per week until 4 normal values are obtained.
Then, obtain them once per month for 1 year. Have
patients use a reliable method of contraception.
In/Out Patient Meds
-
During the period of follow-up care,
patients with gestational trophoblastic disease should
use a reliable method of contraception, such as oral
contraceptives or depot progesterone.
-
The serum HCG titers are critical in
monitoring the status of the disease, and a normal
intrauterine pregnancy interferes with this critical
monitoring tool.
Complications
Prognosis
-
Nonmetastatic gestational trophoblastic
neoplasia has a cure rate with chemotherapy of close to
100%.
-
Metastatic low-risk gestational
trophoblastic neoplasia has a cure rate with
chemotherapy of close to 100%.
-
Metastatic high-risk gestational
trophoblastic neoplasia has a cure rate with
chemotherapy of approximately 75%.
-
After 12 months of normal HCG titers,
less than 1% of patients with malignant gestational
trophoblastic neoplasia have recurrences.
Patient Education
-
The rate of occurrence of a repeat molar
pregnancy is approximately 1-2%.
-
The rate of occurrence of a repeat molar
pregnancy in a patient with a history of 2 previous
hydatidiform moles is approximately 10-20%.
-
The pregnancy rate after chemotherapy
with MTX and cyclophosphamide is 80%. Of women treated
with EMA-CO, 46% have had at least 1 live birth after
chemotherapy.
-
Patients who become pregnant after
treatment for gestational trophoblastic neoplasia should
have a pelvic ultrasound early during the pregnancy to
confirm that the pregnancy is normal.
MISCELLANEOUS
MULTIMEDIA
|
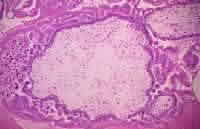
Media file 1: Histological section of a complete
hydatidiform mole stained with hematoxylin and
eosin. Villi of different sizes are present. The
large villous in the center exhibits marked edema
with a fluid-filled central cavity known as
cisterna. Marked proliferation of the trophoblasts
is observed. The syncytiotrophoblasts stain purple,
while the cytotrophoblasts have a clear cytoplasm
and bizarre nuclei. No fetal blood vessels are in
the mesenchyme of the villi. |
|
 |
 View
Full Size Image View
Full Size Image |
|
|
|
Media type: Photo |
|
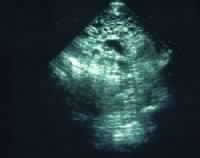
Media file 2: Real-time ultrasound image of a
hydatidiform mole. The dark circles of varying sizes
at the top center are the edematous villi. |
|
 |
 View Full Size Image View Full Size Image |
|
|
|
Media type: Image |
REFERENCES
-
Amir SM, Osathanondh R, Berkowitz RS,
Goldstein DP. Human chorionic gonadotropin and thyroid
function in patients with hydatidiform mole. Am J
Obstet Gynecol. Nov 15 1984;150(6):723-8.
-
Bandy LC, Clarke-Pearson DL, Hammond
CB. Malignant potential of gestational trophoblastic
disease at the extreme ages of reproductive life. Obstet
Gynecol. Sep 1984;64(3):395-9.
-
Barnard DE, Woodward KT, Yancy SG, et
al. Hepatic
metastases of choriocarcinoma: a report of 15 patients. Gynecol
Oncol. Sep 1986;25(1):73-83.
-
Batorfi J, Vegh G, Szepesi J, et al. How
long patients should be followed after molar pregnancy?
Analysis of serum hCG follow-up data. Eur J Obstet
Gynecol Reprod Biol. Jan 15 2004;112(1):95-7.
-
Berkowitz RS, Goldstein DP. Chorionic
tumors. N Engl J Med. Dec 5 1996;335(23):1740-8.
-
Bovicelli L, Ghi T, Pilu G, et al. Prenatal
diagnosis of a complete mole coexisting with a
dichorionic twin pregnancy: case report. Hum Reprod. May 2004;19(5):1231-4.
-
Bower M, Newlands ES, Holden L, et al. EMA/CO
for high-risk gestational trophoblastic tumors: results
from a cohort of 272 patients. J Clin Oncol. Jul 1997;15(7):2636-43.
-
Chauhan S, Diamond MP, Johns DA. A case
of molar ectopic pregnancy. Fertil Steril. Apr 2004;81(4):1140-1.
-
Cheung AN, Khoo US, Lai CY, et
al. Metastatic trophoblastic disease after an initial
diagnosis of partial hydatidiform mole: genotyping and
chromosome in situ hybridization analysis. Cancer. Apr
1 2004;100(7):1411-7. .
-
Escobar PF, Lurain JR, Singh DK, et
al. Treatment of high-risk gestational trophoblastic
neoplasia with etoposide, methotrexate, actinomycin D,
cyclophosphamide, and vincristine chemotherapy. Gynecol
Oncol. Dec 2003;91(3):552-7.
-
FIGO Oncology Committee. FIGO staging for
gestational trophoblastic neoplasia 2000. Int J
Gynaecol Obstet. Jun 2002;77(3):285-7. .
-
Feltmate CM, Genest DR, Goldstein DP,
Berkowitz RS. Advances
in the understanding of placental site trophoblastic
tumor. J Reprod Med. May 2002;47(5):337-41.
-
Feltmate CM, Batorfi J, Fulop V, et al. Human
chorionic gonadotropin follow-up in patients with molar
pregnancy: a time for reevaluation. Obstet Gynecol. Apr 2003;101(4):732-6.
-
Fishman DA, Padilla LA, Keh P, et al. Management
of twin pregnancies consisting of a complete
hydatidiform mole and normal fetus. Obstet Gynecol. Apr 1998;91(4):546-50.
-
Foulmann K, Guastalla JP, Caminet N. What
is the best protocol of single-agent methotrexate
chemotherapy in nonmetastatic or low-risk metastatic
gestational trophoblastic tumors? A review of the
evidence. Gynecol Oncol. Jul 2006;102(1):103-10.
-
Garner EI, Lipson E, Bernstein MR, et
al. Subsequent
pregnancy experience in patients with molar pregnancy
and gestational trophoblastic tumor. J Reprod Med. May 2002;47(5):380-6.
-
Garner EI, Garrett A, Goldstein DP. Significance
of chest computed tomography findings in the evaluation
and treatment of persistent gestational trophoblastic
neoplasia. J Reprod Med. Jun 2004;49(6):411-4.
-
Ghaemmaghami F, Behtash N, Memarpour N,
et al. Evaluation and management of brain metastatic
patients with high-risk gestational trophoblastic
tumors. Int J Gynecol Cancer. Sep-Oct 2004;14(5):966-71. .
-
Goto S, Yamada A, Ishizuka T, Tomoda
Y. Development of post-molar trophoblastic disease after
partial molar pregnancy. Gynecol Oncol. Feb 1993;48(2):165-70.
-
Goto S, Ino K, Mitsui T, et al. Survival
rates of patients with choriocarcinoma treated with
chemotherapy without hysterectomy: effects of anticancer
agents on subsequent births. Gynecol Oncol. May 2004;93(2):529-35.
-
Grimes DA. Epidemiology of gestational
trophoblastic disease. Am J Obstet Gynecol. Oct
1 1984;150(3):309-18.
-
Hammond CB, Weed JC Jr, Currie JL. The
role of operation in the current therapy of gestational
trophoblastic disease. Am J Obstet Gynecol. Apr
1 1980;136(7):844-58.
-
Hancock BW, Tidy JA. Current management
of molar pregnancy. J Reprod Med. May 2002;47(5):347-54.
-
Hassadia A, Gillespie A, Tidy
J. Placental site trophoblastic tumour: clinical
features and management. Gynecol Oncol. Dec 2005;99(3):603-7.
-
Hoekstra AV, Keh P, Lurain JR. Placental
site trophoblastic tumor: a review of 7 cases and their
implications for prognosis and treatment. J Reprod
Med. Jun 2004;49(6):447-52.
-
Homesley HD, Blessing JA, Schlaerth J, et
al. Rapid escalation of weekly intramuscular
methotrexate for nonmetastatic gestational trophoblastic
disease: a Gynecologic Oncology Group study. Gynecol
Oncol. Dec 1990;39(3):305-8.
-
Kajii T, Kurashige H, Ohama K, Uchino
F. XY and XX complete moles: clinical and morphologic
correlations. Am J Obstet Gynecol. Sep
1 1984;150(1):57-64.
-
Kashimura Y, Kashimura M, Sugimori H, et
al. Prophylactic chemotherapy for hydatidiform mole.
Five to 15 years follow-up. Cancer. Aug
1 1986;58(3):624-9.
-
Khanlian SA, Cole LA. Management of
gestational trophoblastic disease and other cases with
low serum levels of human chorionic gonadotropin. J
Reprod Med. Oct 2006;51(10):812-8.
-
Khanlian SA, Cole LA. Management of
gestational trophoblastic disease and other cases with
low serum levels of human chorionic gonadotropin. J
Reprod Med. Oct 2006;51(10):812-8.
-
Kohorn EI. Is lack of response to
single-agent chemotherapy in gestational trophoblastic
disease associated with dose scheduling or chemotherapy
resistance?. Gynecol Oncol. Apr 2002;85(1):36-9.
-
Kwon JS, Elit L, Mazurka J, et al. Weekly
intravenous methotrexate with folinic acid for
nonmetastatic gestational trophoblastic neoplasia. Gynecol
Oncol. Aug 2001;82(2):367-70.
-
Lurain JR, Singh DK, Schink JC. Primary
treatment of metastatic high-risk gestational
trophoblastic neoplasia with EMA-CO chemotherapy. J
Reprod Med. Oct 2006;51(10):767-72.
-
Machtinger R, Gotlieb WH, Korach J, et
al. Placental site trophoblastic tumor: outcome of five
cases including fertility preserving management. Gynecol
Oncol. Jan 2005;96(1):56-61.
-
Mangili G, Garavaglia E, De Marzi P, et
al. Metastatic
placental site trophoblastic tumor. Report of a case
with complete response to chemotherapy. J Reprod Med. Mar 2001;46(3):259-62.
-
Massad LS, Abu-Rustum NR, Lee SS, Renta
V. Poor compliance with postmolar surveillance and
treatment protocols by indigent women. Obstet Gynecol. Dec 2000;96(6):940-4.
-
Matsui H, Iitsuka Y, Suzuka K, et
al. Risk of abnormal pregnancy completing chemotherapy
for gestational trophoblastic tumor. Gynecol Oncol. Feb 2003;88(2):104-7.
-
Matsui H, Iitsuka Y, Suzuka K, et
al. Salvage chemotherapy for high-risk gestational
trophoblastic tumor. J Reprod Med. Jun 2004;
49(6):438-42. .
-
Ngan HY, Chan FL, Au VW, et al. Clinical
outcome of micrometastasis in the lung in stage IA
persistent gestational trophoblastic disease. Gynecol
Oncol. Aug 1998;70(2):192-4.
-
Nigam S, Singhal N, Kumar Gupta S, et
al. Placental site trophoblastic tumor in a
postmenopausal female--a case report. Gynecol Oncol. May 2004;
93(2):550-3.
-
Olsen TG, Hubert PR, Nycum LR. Falsely
elevated human chorionic gonadotropin leading to
unnecessary therapy (1). Obstet Gynecol. Nov 2001;98(5
Pt 1):843-5.
-
Papadopoulos AJ, Foskett M, Seckl MJ, et
al. Twenty-five
years'' clinical experience with placental site
trophoblastic tumors. J Reprod Med. Jun 2002;47(6):460-4.
-
Roberts JP, Lurain JR. Treatment of
low-risk metastatic gestational trophoblastic tumors
with single-agent chemotherapy. Am J Obstet Gynecol. Jun 1996;174(6):1917-23;
discussion 1923-4.
-
Sand PK, Lurain JR, Brewer JI. Repeat
gestational trophoblastic disease. Obstet Gynecol. Feb 1984;63(2):140-4.
-
Schechter NR, Mychalczak B, Jones W,
Spriggs D. Prognosis of patients treated with
whole-brain radiation therapy for metastatic gestational
trophoblastic disease. Gynecol Oncol. Feb 1998;68(2):183-92.
-
Sebire NJ, Fisher RA, Foskett M, et al. Risk
of recurrent hydatidiform mole and subsequent pregnancy
outcome following complete or partial hydatidiform molar
pregnancy. BJOG. Jan 2003;110(1):22-6.
-
Smith HO, Kohorn E, Cole LA. Choriocarcinoma
and gestational trophoblastic disease. Obstet Gynecol
Clin North Am. Dec 2005;32(4):661-84.
-
Soper JT, Evans AC, Conaway MR, et
al. Evaluation of prognostic factors and staging in
gestational trophoblastic tumor. Obstet Gynecol. Dec 1994;84(6):969-73.
-
Soper JT, Clarke-Pearson DL, Berchuck A,
et al. 5-day methotrexate for women with metastatic
gestational trophoblastic disease. Gynecol Oncol. Jul 1994;54(1):76-9.
-
Soto-Wright V, Bernstein M, Goldstein DP,
Berkowitz RS. The changing clinical presentation of
complete molar pregnancy. Obstet Gynecol. Nov 1995;86(5):775-9.
-
Suzuka K, Matsui H, Iitsuka Y, et al. Adjuvant
hysterectomy in low-risk gestational trophoblastic
disease. Obstet Gynecol. Mar 2001;97(3):431-4.
-
Tidy JA, Gillespie AM, Bright N, et
al. Gestational trophoblastic disease: a study of mode
of evacuation and subsequent need for treatment with
chemotherapy. Gynecol Oncol. Sep 2000;78(3 Pt
1):309-12.
-
Xiang Y, Sun Z, Wan X, Yang X. EMA/EP
chemotherapy for chemorefractory gestational
trophoblastic tumor. J Reprod Med. Jun 2004;49(6):443-6.
|
|