|
I HAVE RECENTLY READ - Pregnancy-related hypertensive disorders and risk for cardiovascular disease MRI
Diagnosis of Accreta Introduction There is no doubt that ultrasound is the principal imaging tool for the detection and evaluation of placenta accreta as described elsewhere in this volume. Magnetic resonance imaging (MRI) was first used in pregnancy soon after it was developed, and those early imaging attempts paid particular attention to the placenta. At the time, availability of probes for endovaginal ultrasound was limited and researchers and clinicians sought other modalities to evaluate placental location, typically to diagnose placenta previa. Without diagnostic imaging, women with a suspected placenta previa in the late third trimester underwent vaginal examination in an operating room under a “double set-up” prepared for cesarean section. If placenta previa was present, delivery by cesarean section was immediately performed; if not, labor was induced. MRI was an effective tool for placental localization, but was never widely adopted as endovaginal probes became readily available. Later investigators and clinicians turned their attention to MRI as a tool for diagnosis and evaluation of accreta, often with mixed results. In contemporary practice, MRI has a role in the armamentarium of tools used in the diagnosis and management of placenta accreta although its precise application remains controversial. MRI Technique Obstetric MRI has evolved over the years with different techniques being proposed and adopted depending on the type of investigation being performed. Fetal MRI uses protocols that vary according to the anatomic area of interest and must be modified to reduce movement artefact and allow evaluation of small details of fetal anatomy. Protocols used in placental evaluation have shown a similar pattern of evolution. Although differences exist between individual centres and clinicians, the approach for most studies has become relatively uniform. At our institution, MR exams are performed using a 1.5-Tesla MRI system with a phased-array torso coil. Patients are imaged without sedation and usually fast for 4 hours prior to imaging because an empty GI tract produces better images. Patients are positioned supine or decubitus based on patient comfort and gestational age. Initial pulse sequences rely on the ultrafast T2-weighted single-shot fast spin echo (SSFSE/HASTE), T2/T1-weighted balanced steady-state free precession (FISP/FIESTA), and T1-weighted gradient spin echo images. Sequences are acquired in at least two orthogonal planes over the region of interest, most commonly the lower uterine segment. High-resolution T2-weighted echo train and spin echo may be helpful when focused upon areas of particularly high suspicion. Gadolinium-based contrast is administered intravenously only if unenhanced images are indeterminate to confirm the diagnosis and assess depth of invasion if the gestational age is 28 weeks or greater. One-half dose of MultiHanceR (gadobenate dimeglumine, Bracco Diagnostics Inc., Singen, Germany) is typically sufficient. Dynamic sequences are acquired using two-dimensional (2D) gradient echo, MR angiographic sequences, or modified MR angiographic sequences that speed acquisition time (e.g., time-resolved imaging of contrast kineticS [TRICKS]) to eliminate the need for timing. All patients provide informed consent prior to the exams for both MRI and intravenous contrast administration. A radiologist monitors all studies in order to adequately evaluate the area of interest. MRI and Safety in Pregnancy MRI is generally considered to be safe in pregnancy. It does not use ionizing radiation, appears to have no risk of teratogenicity, and does not require special restrictions or precautions. There are no published studies documenting harm in human or animal use. Theoretical safety concerns have been raised regarding the use of higher strength magnetic fields (3-Tesla MRI), although no data exist to support evidence of harm. Pregnant patients may require left lateral positioning to reduce caval compression during scanning. MRI provides good differentiation between tissues without the use of contrast. However, the use of contrast has been advocated in some settings including the evaluation of placenta accreta. There are no data supporting the use or safety of super paramagnetic iron oxide particles in pregnancy and only gadolinium-based contrast should be used. However, the use of gadolinium in pregnancy is controversial. Gadolinium has not been shown to be teratogenic or mutagenic in animal studies and did not have adverse fetal or new-born effects when used in the first trimester in pregnant women. Free gadolinium is toxic and should only be administered in chelated form; the stability of available compounds varies and it has been suggested that only the most stable compounds be used in pregnancy. Gadolinium crosses the placenta to enter the fetal circulation, is excreted into fetal urine, swallowed as amniotic fluid, is recirculated, and can pass via the placenta to the maternal circulation for final excretion. Concerns have been raised regarding a potential risk of nephrogenic systemic fibrosis (NSF) in neonates exposed to gadolinium in utero because of relatively low fetal and new-born glomerular filtration rate (GFR). An elegant recent study in nonhuman pregnant primates examined the persistence of gadolinium in amniotic fluid and accumulation of gadolinium in fetal tissues following a clinically relevant dose of contrast. The authors found minimal residual gadolinium in amniotic fluid 21 hours after injection and evidence of continuing excretion. Despite these reassuring findings, current recommendations are to restrict gadolinium use to the most stable agents and to use contrast only when benefits clearly outweigh potential risk. We believe it is a reasonable option in the third trimester. MRI and Normal Placenta It is essential that one can identify
the appearance of normal placenta before attempting to
evaluate abnormal placentation. It also is important to
consider the changes that occur in placental anatomy over
the course of normal pregnancy. The pregnant uterus is pear
shaped with a narrower lower segment than the fundus and
body. The placenta may be implanted anteriorly or
posteriorly and wrap laterally. The lower edge of the
placenta is easily visualized, making assessment of the
distance between the placental and cervix relatively easy.
On T1-weighted images, the placenta appears homogeneous and
is isointense to muscle throughout pregnancy. Thus, it is
difficult to examine the placental–uterine interface or the
myometrium (Figure 4.1a). Fat-suppressed T2-weighted imaging
also shows the placenta to be bright and homogeneous in
texture with darker thin septae and vessels (Figure 4.1b).
The fetal and maternal surfaces of the placenta appear
smooth. In the third trimester, the inner fetal surface of
the placenta may become lobulated in a pattern related to
maturation of the placental cotyledons. The outer maternal
surface should remain smooth, following the contours of the
uterine wall. When contrast is used, the placenta enhances
prior to the myometrium and with increasing age becomes more
homogeneous. Normal myometrium has a three-layered
appearance on T2-weighted images with a heterogeneous
hyperintense middle layer and low-intensity margins (Figure
4.1b). As pregnancy progresses, the myometrium thins as the
uterus enlarges. MRI and Placenta Accreta Over the last 20 years, numerous authors have described MRI features, which suggest possible placenta accreta.22–28 These include a heterogeneous appearance of the placenta with linear T2-hypointense intraplacental bands, abnormal uterine segment bulging, disruption of the hypo intense bladder wall and a nodular appearance of the bladder outline with placental extension into the bladder. Figure 4.2 shows a placenta with features of accreta. T2-weighted images are shown in sagittal (a) and coronal (b) planes demonstrating a heterogeneous disorganized placental signal with dark T2 bands, an irregular, lobular outer contour, and bladder indentation. Obtaining additional sequences may allow concerning features to be further evaluated. Figure 4.3 shows a case with a posterior placenta previa without accreta. T2-weighted SSFSE images show the interface between the placenta and uterine wall. On T2-weighted images, dark linear structures may represent normal intra-placental vessels or the dark bands associated with accreta.
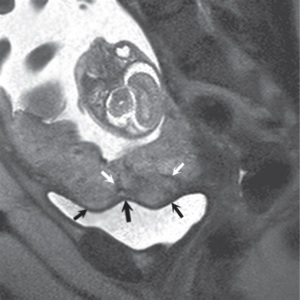
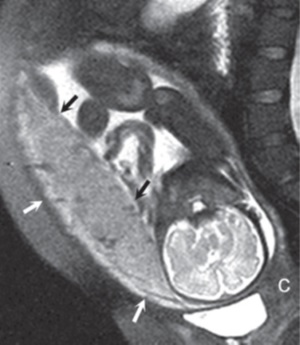
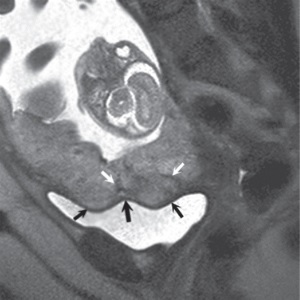
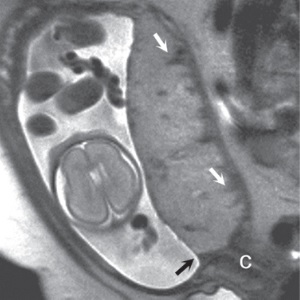
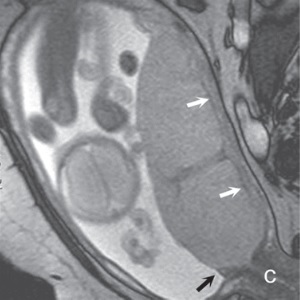
(a) (b) FIGURE 4.1 Normal placenta on magnetic resonance imaging (MRI) in the late second trimester. (a) On T1-weighted images, the placenta is homogeneous and isointense to muscle throughout pregnancy. (b) On fat-suppressed T2-weighted images, the placenta is fairly homogeneous and bright with darker thin septa and vessels seen. Note both the inner (fetal) surface (white arrows, a; black arrows, b) and outer (maternal) surface (black arrows, a; white arrows, b) of the placenta are smooth. There is no placenta previa in this case as the anterior placenta is remote from the cervix (“c,” a and b). Later in the third trimester, the inner surface may become lobulated in a regular pattern related to the cotyledons; however, the outer maternal surface remains smooth, flowing the expected contour of the uterine wall. Often, a T2/T1-weighted steady-state gradient echo sequence helps distinguish between placental bands and vessels; placental bands appear dark on this sequence and vessels appear bright. The placental–uterine interface is often better seen on T2/T1-weighted steady-state gradient echo, appearing as a dark line separating the placenta from the uterine wall. The vascularity of the uterine wall frequently leads to low signal intensity on T2-weighted images and higher signal intensity on FISP/FIESTA images.
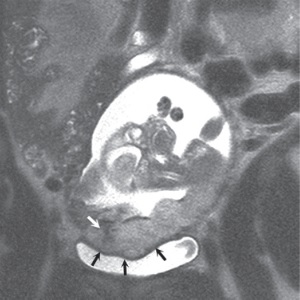
(a) (b) FIGURE 4.2 Placenta accreta. Sagittal (a) and coronal (b) T2-weighted images show heterogeneous, disorganized signal, dark T2 bands (white arrows, a and b), and irregular, lobular, outer contour (black arrows, a and b) indenting the partially distended bladder.
(a) (b) FIGURE 4.3 Posterior placenta previa
with no accreta: value of FISP/FIESTA sequence. (a) The
T2-weighted SSSFE/ HASTE shows fairly a homogeneous placenta
previa with normal but indistinct interface with the uterine
wall. In addition, dark linear structures are seen that may
be confused with dark bands of accreta (white arrows, a).
(b) On the T2/ T1-weighted steady-state gradient echo
(FISP/FIESTA), the dark bands are not seen (suggesting they
are vascular structures), and it is easier to distinguish
placental–maternal interface, which may be seen as a thin
dark line (white arrows, b) separating the placenta from the
uterine wall. The vascularity of the uterine wall frequently
leads to low signal intensity on T2-weighted images and
higher signal intensity on FISP/FIESTA images. The anterior
placental edge is seen (black arrow, a and b), covering the
internal orifice (os) of the cervix (“c,” a and b). MRI and Placenta Accreta and Gestational Age at Evaluation There is general consensus that MRI to evaluate fetal anatomy is of little use at gestational ages of less than 18 weeks because of the small fetal size and high rate of motion artefact. MRI is of greater use at 20–22 weeks or later depending on the area of interest.7 An awareness that placenta accreta may be diagnosed with ultrasound as early as the first trimester has led to MRI being performed at earlier and earlier gestational ages for confirmation of ultrasound suspected diagnoses. A recent study examined the diagnostic utility of MRI performed at 14–41 weeks gestation. The authors showed that when MRI was performed at less than 24 weeks, the sensitivity and specificity for a diagnosis of placenta accreta was 0.14 and 0.7, respectively. This was considerably worse than the values of 0.79 and 0.94 obtained after 24 weeks. The positive predictive value (PPV) and negative predictive value (NPV) in studies at less than 24 weeks gestation (0.25 and 0.54, respectively) were also worse than after 24 weeks (0.96 and 0.71, respectively). They concluded that MRI evaluation should be delayed until after 24 weeks gestation. MRI or Ultrasound for Screening and Initial Diagnosis of Placenta Accreta Several studies examined the performance of MRI versus ultrasound as the primary diagnostic modality for diagnosing placenta accreta. Ultrasound and MRI performed similarly for primary diagnosis in all studies with no differences found in sensitivity or specificity between the imaging techniques (MRI sensitivity 92.9%, US sensitivity 87.8%, p = 0.24; MRI specificity 93.5%, US specificity 96.3%, p = 0.91). Given the relative availability, ease of performance, and lower cost of ultrasound versus MRI, it follows that ultrasound should be the primary screening tool for accreta in at-risk patients. MRI and Antepartum Hemorrhage Bleeding in pregnancy should be
evaluated initially with ultrasound. This is particularly
true in the second half of pregnancy where placenta previa
should always be excluded prior to vaginal examination. In
cases where accreta is suspected in the setting of bleeding
in a hemodynamically stable patient, MRI may have a
diagnostic role. Figure 4.4a shows a sagittal T2-weighted
image of a placenta accreta with outer bulging of the
placenta, and Figure 4.4b shows a T2/T1-weighted FISP/FIESTA
image. Both figures suggest that there is placental tissue
at the internal orifice (os). The T1-weighted image in
Figure 4.4c clearly shows that this apparent placental
tissue is an area of hemorrhage, demonstrating the value of
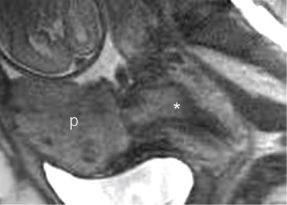
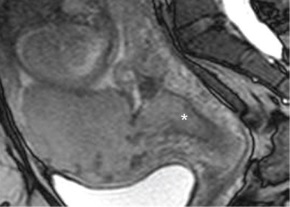
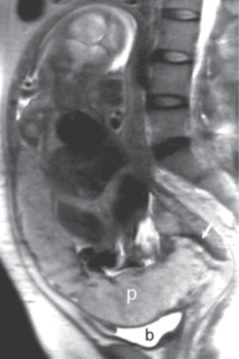
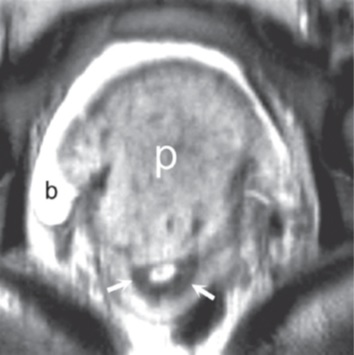
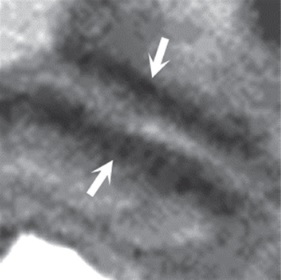
such additional sequences. MRI for Assessment of Depth of Myometrial Invasion The gold standard for assessment of the depth of placental invasion is histopathology. It should be noted, however, that in a single specimen, varying degrees of placental invasion from accreta, increta, and percreta may occur, depending on the site examined. Ultrasound has been shown to have a wide range of diagnostic confidence in determining degree of placental invasion from 38% to 65%. Figure 4.5a shows a sagittal T2-weighted image of placenta accreta with outer bulging of the placenta and invasion into the cervix. Figure 4.5b shows the axial T2-weighted view (oriented axial to the cervix) with the dark inner fibrous stromal ring of the cervix replaced by the invasive placenta anteriorly. Figures 4.5c and 4.5d show a normal intact appearance of the dark fibrous stroma in a normal cervix. The previously cited meta-analysis of MRI and placenta accreta35 found a sensitivity of 92.9% (72.8%–99.5%) and a specificity of 97.6% (87.1%–99.9%) for depth of myometrial invasion. Palacios-Jaraquemada described a system of classifying degree of placental invasion coupled with topographic assessment of the placenta in 300 cases and reported accurate assessment of placental invasion in 97.66% of cases with a 1.33% false positive rate and 1% false negative rate. This system was applied retrospectively to 62 patients managed at a single centre. Partial myometrial invasion was graded A, invasion to full thickness of myometrium B, and invasion into parametrium or cervix C. The location of invasion was classified as S1, upper uterine segment supplied by uterine and upper vesical arteries, and S2, lower uterine segment supplied by deep anastomotic pelvic subperitoneal vessels. Severity ranged from S1A (least severe) to S2C (most severe). MRI assessment was compared to pathological and surgical staging. The authors reported a 61% rate of accuracy for staging, with a 22.6% rate of over diagnosis of degree of invasion. Further prospective studies are needed to examine the true utility of this approach.
(a) (b)
(c) (FIGURE 4.4 Placenta accreta with hemorrhage: value of T1-weighted sequence. (a) Sagittal T2-weighted image shows placenta accreta (“p,” a) with outer bulging of the placenta. Note the material at the internal Os has the appearance of placental tissue on both T2-weighted (a) and T2/T1-weighted FISP/FIESTA (b) images (“*,” a & b). On the T1-weighted image (c), we see there is hemorrhage in this location (arrows, c). This case demonstrates the importance of a T1-weighted sequence to clearly identify blood products that may mimic solid tissue on other sequences. MRI as a Tie Breaker in Uncertain Diagnosis of Accreta In cases where it is difficult to determine if an accreta is present, MRI may be useful as an adjunctive diagnostic test. This may be particularly true in cases of posterior placentation, especially in later gestation when it is difficult to visualize the placenta by sonogram, or suspected accreta absent of placenta previa. Such difficult cases may also benefit from the use of contrast media.
(a) (b)
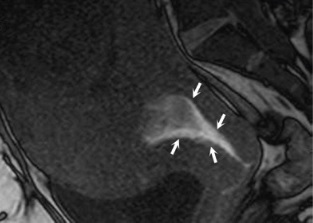
(c) (d) FIGURE 4.5 Placenta accreta with
cervical invasion. (a) Sagittal T2-weighted image shows
placenta accreta with outer bulging of the placenta (“p,” a)
and invasion into the cervix; only the posterior dark stripe
of normal fibrous stroma is seen (arrow, a). (b) On the
axial T2-weighted view, the dark inner fibrous stromal ring
(arrows, b) of the cervix is partially replaced by the
anterior invasive placenta (“p,” b). Note relationship of
the placenta with fluid in the bladder (“b,” a and b). (c)
For comparison, sagittal (c) and axial (d) T2-weighted
images indicate the normal intact appearance of the dark
fibrous stroma of the cervix (arrows, c and d). (Gratitude: This academic material has been excerpted with deep gratitude from the book Placenta Accreta Syndrome edited by Robert Silver and published by CRC Press, Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487-2742. There is no financial or any other material gain from this excerpt and is being reproduced here only with a benevolent aim to help the readers to know more about the potentially lethal condition of placenta accreta syndrome. Readers wishing to read the complete article including the complete comprehensive top-class material should refer to the book cited above. Once again a deep gratitude to the editor, the authors and the publishers for this material.)
|
|||||||||||||||